Practice CBSE Class 10 Science Chemical Reactions and Equations MCQs Set D provided below. The MCQ Questions for Class 10 Chapter 1 Chemical Reactions and Equations Science with answers and follow the latest CBSE/ NCERT and KVS patterns. Refer to more Chapter-wise MCQs for CBSE Class 10 Science and also download more latest study material for all subjects
MCQ for Class 10 Science Chapter 1 Chemical Reactions and Equations
Class 10 Science students should review the 50 questions and answers to strengthen understanding of core concepts in Chapter 1 Chemical Reactions and Equations
Chapter 1 Chemical Reactions and Equations MCQ Questions Class 10 Science with Answers
Question: How many water molecules are present in a crystal of copper sulphate molecule?
a) 5
b) 7
c) 2
d) 3
Answer: a
Question: CuO + H2 → H2O + Cu, reaction is an example of –
a) redox reaction
b) synthesis reaction
c) neutralisation
d) analysis reaction
Answer: a
Question: Which of the following chemical equations is an unbalanced one ?
a) 2NaHCO3 → Na2CO3 + H2O + CO2
b) 2C4H10 + 12O2 → + 8CO2 + 10H2O
c) 2Al + 6H2O →2Al(OH)3 + 3H2
d) 4NH3 + 5O2 → 4NO + 6H2O
Answer: b
Question: Which of the following gases can be used for storage
a) Carbon dioxide or Oxygen
b) Nitrogen or Oxygen
c) Carbon dioxide or Helium
d) Helium or Nitrogen
Answer: d
Question: Which of the following can be decomposed by the action of light?
a) NaCl
b) KCl
c) AgCl
d) CuCl
Answer: c
Question: Which among the following is not a physical change?
a) Melting of solid to liquid.
b) Vaporisation of liquids to gas.
c) Liquefaction of gases to liquid.
d) Decay of matter.
Answer: d
Question: How many water molecules are present in a crystal of ferrous sulphate molecule?
a) 5
b) 2
c) 7
d) 10
Answer: c
Question: Which of the following is not a physical change?
a) Boiling of water to give water vapour.
b) Melting of ice to give water.
c) Dissolution of salt in water.
d) Combustion of Liquefied Petroleum Gas (LPG).
Answer: d
Question:Which of the following is a thermal decomposition reaction?
a) 2H2O → 2H2+ O2
b) 2AgCl → 2Ag + Cl2
c) H2(g) + Cl2(g) → 2HCl(g)
d) ZnCO3→ ZnO + CO2
Answer: d
Question: Reaction between calcium oxide and water is a ______________ reaction.
a) endothermic reaction
b) decomposition reaction
c) exothermic reaction
d) displacement reaction
Answer: c
Question: Which of the following stands for a double displacement reaction ?
a) 2H2 + O2 → 2H2O
b) 2Mg + O2 →2MgO
c) AgNO3 + NaCl → AgCl + NaNO3
d) H2 + Cl2 → 2HCl
Answer: c
Question: The schematic diagram is given below
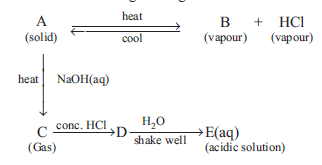
Which of the following is a correct statement ?
a) A and E are chemically same.
b) A and D are chemically same.
c) D and E are chemically same.
d) C and E are chemically same.
Answer: b
Question: The reaction, H2 + Cl2 →2HCl is
a) an oxidation reaction
b) a reduction reaction
c) a combination reaction
d) an isomerisation reaction
Answer: c
Question: Decomposition of water is
a) electrolytic
b) thermal
c) photolytic
d) All of the above
Answer: a
Question: When the gases sulphur dioxide and hydrogen sulphide mix in the presence of water, the reaction is SO2 + 2H2S → 2H2O + 2S. Here hydrogen sulphide is acting as –
a) an oxidising agent
b) a reducing agent
c) a dehydrating agent
d) a catalyst
Answer: b
Question:When green coloured ferrous sulphate crystals are heated, the colour of the crystal changes because:
a) it is decomposed to ferric oxide
b) it loses water of crystallisation
c) it forms SO2
d) it forms SO3
Answer: b
Question: On the basis of following features, identify the correct option.
(i) This reaction occurs during corrosion.
(ii) This reaction occurs during respiration.
a) Decomposition reaction
b) Redox reaction
c) Combination reaction
d) Endothermic reaction
Answer: b
Question: Which of the following is a displacement reaction ?
a) CaCO3 → CaO + CO2
b) CaO + 2HCl →CaCl2 + H2O
c) Fe + CuSO4 → FeSO4 + Cu
d) NaOH + HCl →NaCl + H2O
Answer: c
Question: Which of the following is a displacement reaction?
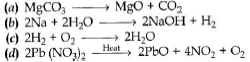
Answer: b
Question:The condition produced by aerial oxidation of fats and oils in foods marked by unpleasant smell and taste is called:
a) Antioxidation
b) Reduction
c) Rancidity
d) Corrosion
Answer: c
Question: Chemical changes are __________.
a) temporary, reversible and a new substance is produced.
b) always accompanied by exchange of light.
c) permanent, irreversible and a new substance is produced.
d) never accompanied by exchange of light and heat energy.
Answer: c
Question: Lead nitrate on heating gives
a) Lead oxide
b) Nitrogen dioxide
c) Oxygen
d) All of these
Answer: d
Question: Which of the following equations is representing combination of two compounds ?
a) CaO + CO2¾→ CaCO3
b) CO +1/2O2 ¾→ CO2
c) SO2 +1/2O2 ¾→ SO3
d) 2Na + 2H2O ¾→ 2NaOH + H2
Answer: a
Question: Fe2O3 + 2Al ⎯⎯→ Al2O3 + 2Fe
The above reaction is an example of a:
a) combination reaction
b) double displacement reaction
c) decomposition reaction
d) displacement reaction
Answer: d
Question: What is the colour of FeSO4.7H2O?
a) Blue
b) Green
c) White
d) Brown
Answer: b
Question: When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of –
a) a combination reaction
b) a displacement reaction
c) a decomposition reaction
d) a double decomposition reaction
Answer: d
Question: Which of the following is not a decomposition reaction ?
a) CaCO3 → CaO + CO2
b) 2KClO3 →2KCl + 3O2
c) Digestion of food in body
d) H2 + Cl2 →2HCl
Answer: d
Question: In which of the following the identity of initial substance remains unchanged?
a) Curdling of milk
b) Formation of crystals by process of crystallisation
c) Fermentation of grapes
d) Digestion of food
Answer: b
Question: Which of these will cause a chemical change to occur ?
a) Grinding of wheat into flour.
b) Lighting of a gas stove.
c) Evaporation of water from a lake.
d) Ringing of an electric bell.
Answer: b
Question: A balanced chemical equation is in accordance with –
a) Avogadro’s law
b) law of multiple proportion
c) law of conservation of mass
d) law of gaseous volumes.
Answer: c
Question: Physical changes are usually _______ in nature.
a) temporary
b) permanent
c) irreversible
d) endothermic
Answer: a
Question: What happens when copper rod is dipped in iron sulphate solution?
a) Copper displaces iron
b) Blue colour of copper sulphate solution is obtained
c) No reaction takes place
d) Reaction is exothermic
Answer: c
Question: Which of the following is a decomposition reaction?
a) 2HgO Heat→2Hg + O2
b) CaCO3 Heat→CaO + CO2
c) 2H2O Electrolysi →H2 + O2
d) All of these
Answer: d
Question: Pb + CuCl2 → PbCl2 + Cu The above reaction is an example of:
a) combination
b) double displacement
c) decomposition
d) displacement
Answer: d
Question:. Magnesium ribbon is rubbed before burning because it has a coating of
a) basic magnesium carbonate
b) basic magnesium oxide
c) basic magnesium sulphide
d) basic magnesium chloride
Answer: b
Question:PbS reacts with ozone (O3) and forms pbso4 . As per the balanced equation, molecules of ozone required for every one molecule of PbS is/are
a) 4
b) 3
c) 2
d) 1
Answer: a
Question: Which of the following is an endothermic reaction?
a) CaCO3 heat CaO + CO2
b) CaO + H2O Ca(OH)2
c) C6H12O6 + O2 6CO2 + 6H2O
d) None of these
Answer: a
Question: In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
a) Lead sulphate (insoluble)
b) (&) Lead acetate
c) Ammonium nitrate
d) Potassium sulphate
Answer: b
Question: Select the oxidising agent for the following reaction:
H2S + I2 > 2HI + S
a) I2
b) H2S
c) HI
d) S
Answer: a
Question: In the equation Cu + xHNO3 → Cu(NO3)2 + yNO2 + 2H2O The values of x and y are
a) 3 and 5
b) 8 and 6
c) 4 and 2
d) 7 and 1
Answer: c
Question: Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is:
a) 1 : 1
b) 2:1
c) 4:1
d) 1:2
Answer: b
Assertion-Reason Questions
In the following question, a statement of Assertion
a) is followed by a statement of Reason (R). Answer these questions by selecting appropriate option given below:
a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
b) Both Assertion (A) and Reason (R) are true but Reason (R) is not the correct explanation of Assertion (A)
c) Assertion (A) is true but Reason (R) is false.
d) Assertion (A) is false but Reason (R) is true.
Question: Assertion (A): Pungent smelling gas is produced when sulphur burns in air.
Reason (R): Sulphur trioxide is formed on reaction of sulphur with oxygen.
Answer: c
Question: Assertion (A): To dilute sulphuric acid, acid is added to water and not water to acid.
Reason (R): Specific heat of water is quite large.
Answer: a
Question: Assertion (A): Calcium Carbonate when heated gives calcium oxide and water.
Reason (R): On heating CaCO3, decomposition reaction takes place.
Answer: d
Question: Assertion (A): Sodium metal is stored under Kerosene.
Reason (R): Metallic sodium melts when exposed to air.
Answer: c
Question: Assertion (A): A reducing agent is a substance which can either accept electron.
Reason (R): A substance which helps in oxidation is known as reducing agent.
Answer: d
Question: Assertion (A): Quicklime reacts vigorously with water releasing a large amount of heat.
Reason (R): The above chemical reaction is an exothermic reaction.
Answer: a
Question: Assertion (A): Photosynthesis is considered as an endothermic reaction.
Reason (R): Energy gets released in the process of photosynthesis
Answer: c
Question: Assertion (A): Fe2O3 + 2Al → Al2O3 + 2Fe The above chemical equation is an example of displacement reaction.
Reason (R): Aluminium being more reactive than iron, displaces Fe from its oxide.
Answer: a
Question: Assertion (A): Stannous chloride is a powerful oxidising agent which oxidises mercuric chloride to mercury.
Reason (R): Stannous chloride gives grey precipitate with mercuric chloride, but stannic chloride does not do so.
Answer: c
Question: Assertion (A): Carbon dioxide turns lime water milky.
Reason (R): Carbon dioxide sullies the water.
Answer: c
Very Short Answer Type Questions
Question: What happens when quicklime is added to water?
Answer: Quicklime reacts with water vigorously to produce slaked lime and a large amount of heat.
Question: State one basic difference between a physical change and a chemical change.
Answer: In a physical change, no new substance is formed. In a chemical change, a new substance is formed.
Question: Which one is a chemical change-rusting of iron or melting of iron?
Answer: Rusting of iron.
Question: What happens when ZnCO3 is heated in the absence of air? Give the relevant equation.
Answer: ZnO(s) and CO2(g)CO2(g) are formed. Chemical Equation:
ZnCO3 → ZnO + CO2
Question: Name and state the law which is kept in mind while we balance a chemical equation.
Answer: Law of conservation of mass. Mass can neither be created nor be destroyed during a chemical reaction.
More free study material for Science
MCQs for Chapter 1 Chemical Reactions and Equations Science Class 10
Students can use these MCQs for Chapter 1 Chemical Reactions and Equations to quickly test their knowledge of the chapter. These multiple-choice questions have been designed as per the latest syllabus for Class 10 Science released by CBSE. Our expert teachers suggest that you should practice daily and solving these objective questions of Chapter 1 Chemical Reactions and Equations to understand the important concepts and better marks in your school tests.
Chapter 1 Chemical Reactions and Equations NCERT Based Objective Questions
Our expert teachers have designed these Science MCQs based on the official NCERT book for Class 10. We have identified all questions from the most important topics that are always asked in exams. After solving these, please compare your choices with our provided answers. For better understanding of Chapter 1 Chemical Reactions and Equations, you should also refer to our NCERT solutions for Class 10 Science created by our team.
Online Practice and Revision for Chapter 1 Chemical Reactions and Equations Science
To prepare for your exams you should also take the Class 10 Science MCQ Test for this chapter on our website. This will help you improve your speed and accuracy and its also free for you. Regular revision of these Science topics will make you an expert in all important chapters of your course.
You can get most exhaustive CBSE Class 10 Science Chemical Reactions and Equations MCQs Set D for free on StudiesToday.com. These MCQs for Class 10 Science are updated for the 2025-26 academic session as per CBSE examination standards.
Yes, our CBSE Class 10 Science Chemical Reactions and Equations MCQs Set D include the latest type of questions, such as Assertion-Reasoning and Case-based MCQs. 50% of the CBSE paper is now competency-based.
By solving our CBSE Class 10 Science Chemical Reactions and Equations MCQs Set D, Class 10 students can improve their accuracy and speed which is important as objective questions provide a chance to secure 100% marks in the Science.
Yes, Science MCQs for Class 10 have answer key and brief explanations to help students understand logic behind the correct option as its important for 2026 competency-focused CBSE exams.
Yes, you can also access online interactive tests for CBSE Class 10 Science Chemical Reactions and Equations MCQs Set D on StudiesToday.com as they provide instant answers and score to help you track your progress in Science.

