Practice CBSE Class 10 Chemistry Periodic Classification of Elements MCQs Set D provided below. The MCQ Questions for Class 10 Chapter 5 Periodic Classification of Elements Science with answers and follow the latest CBSE/ NCERT and KVS patterns. Refer to more Chapter-wise MCQs for CBSE Class 10 Science and also download more latest study material for all subjects
MCQ for Class 10 Science Chapter 5 Periodic Classification of Elements
Class 10 Science students should review the 50 questions and answers to strengthen understanding of core concepts in Chapter 5 Periodic Classification of Elements
Chapter 5 Periodic Classification of Elements MCQ Questions Class 10 Science with Answers
Question: The pairs of elements with the following atomic numbers have the same chemical properties:
a) 13 & 12
b) 3 & 11
c) 4 & 24
d) 2 & 1
Answer: b
Question: Which of the following will form acidic oxide? An element with atomic number:
a) 7
b) 11
c) 21
d) 19
Answer: a
Question: Element with atomic number 15 and mass number 31 is present in:
a) Group 5 and period 4
b) Group 5 and period 3
c) Group 15 and period 3
d) Group 15 and period 4
Answer: c
Question: Where would you locate an element with electronic configuration 2, 8, 7 in the modern periodic table?
a) Group 7 and period 2
b) Group 7 and period 3
c) Group 17 and period 3
d) Group 17 and period 3
Answer: c
Question: The longest and the shortest periods are:
a) 1 & 6
b) 2 & 6
c) 6 & 1
d) 1 & 7
Answer: b
Question: Which amongst the following represents the correct order of decreasing metallic character of elements Na, Si, Cl, Mg, Al?
a) Cl > Si > Al > Mg > Na
b) Na > Mg > Al > Si > Cl
c) Na > Si > Mg > Al > Cl
d) Al > Na > Si > Cl > Mg
Answer: b
Question: Which of the given elements A, B, C, D and E with atomic numbers 2, 4, 8, 10 and 18 respectively belong to the same period?
a) A, B, C
b) B, C, D
c) A, D, E
d) B, D, E
Answer: b
Question: The number of elements present in the 2nd, 3rd, 4th and 5th periods of the modern periodic table are:
a) 2, 8, 8, 18
b) 8, 8, 18, 32
c) 8, 8, 18, 18
d) 8, 18, 18, 32
Answer: b
Question: The period that contains only gaseous elements is:
a) 1
b) 2
c) 3
d) 4
Answer: a
Question: Which of the following are characteristics of isotopes of an element?
a) Isotopes of an element have same atomic masses
b) Isotopes of an element have same atomic number
c) Isotopes of an element show same physical properties
d) Isotopes of an element have same chemical properties
i) A, C, D
ii) B, C, D
iii) B and C
iv) B and D
Answer: c
Question: Which of the following elements has the least nonmetallic character?
a) Fluorine
b) Chlorine
c) Bromine
d) Iodine
Answer: d
Question: The lightest metal is:
a) Li
b) Na
c) K
d) Mg
Answer: a
Question: Which of the following hydroxides is most basic?
a) Be(OH)₂
b) Mg(OH)₂
c) Ca(OH)₂
d) Ba(OH)₂
Answer: d
Question: Which of the following is the correct order of size?
a) Cl < F < Br < I
b) F < Cl < Br < I
c) I < Br < Cl < F
d) Br < I < Cl < F
Answer: b
Question: Why are the elements lithium, sodium and potassium called alkali metals?
a) Because they react with water to form alkali
b) Because they form acidic oxides
c) Because they are present in first group
d) Because they are less reactive in nature
Answer: a
Question: In the periodic table, the metallic character of elements:
a) Decreases from left to right and down the group
b) Decreases from left to right and increases down the group
c) Increases from left to right and down the group
d) Increases from left to right and decreases down the group
Answer: b
Question: The alkali metals are in which group of the periodic table?
a) Group 1
b) Group 2
c) Group 3
d) Group 4
Answer: a
Question: The most metallic element in the fourth period is:
a) Ca
b) K
c) S
d) P
Answer: b
Question: Which of the following elements would accept an electron readily?
a) F
b) Cl
c) Br
d) I
Answer: b
Question: Which of the following elements will form acidic oxide?
a) Sodium
b) Magnesium
c) Aluminium
d) Sulphur
Answer: d
Question: Which of the following elements would lose an electron easily?
a) K
b) Na
c) Ca
d) Mg
Answer: b
Question: The correct order of size among I⁺, I⁻ and I is:
a) I⁺ > I⁻ > I
b) I⁻ > I > I⁺
c) I > I⁺ > I⁻
d) I > I⁻ > I⁺
Answer: b
Question: An element has 13 protons. The group and period to which it belongs:
a) 3rd period and 13th group
b) 2nd period and 13th group
c) 3rd period and 3rd group
d) 2nd period and 3rd group
Answer: a
Question: Which of the following hydroxides is most basic?
a) Be(OH)₂
b) Ba(OH)₂
c) Ca(OH)₂
d) Mg(OH)₂
Answer: b
Question: According to Mendeleev’s periodic law, the properties of elements are a periodic function of their:
a) Atomic number
b) Atomic masses
c) Atomic volumes
d) Atomic sizes
Answer: b
Question: Gradual addition of electronic shells in the noble gases causes a decrease in their:
a) Ionisation energy
b) Atomic radius
c) Boiling point
d) Density
Answer: a
Question: "The properties of the elements are periodic function of their atomic numbers." The statement was given by:
a) N. Bohr
b) J.W. Dobereiner
c) D.I. Mendeleev
d) H.G.J. Moseley
Answer: d
Question: Which of the following has most non-metallic character?
a) N
b) C
c) O
d) F
Answer: d
Question: Cl, Br, I – if this is a Dobereiner's triad and the atomic masses of Cl and I are 35.5 and 127 respectively, the atomic mass of Br is:
a) 162.5
b) 91.5
c) 81.25
d) 45.625
Answer: c
Question: Which pair of elements of the following sets is likely to have similar chemical behaviour?
a) Sodium and aluminium
b) Argon and potassium
c) Boron and germanium
d) Nitrogen and phosphorus
Answer: d
Question: Which of the following oxides is amphoteric in nature?
a) CaO
b) CO₂
c) SiO₂
d) SnO₂
Answer: d
Case study questions
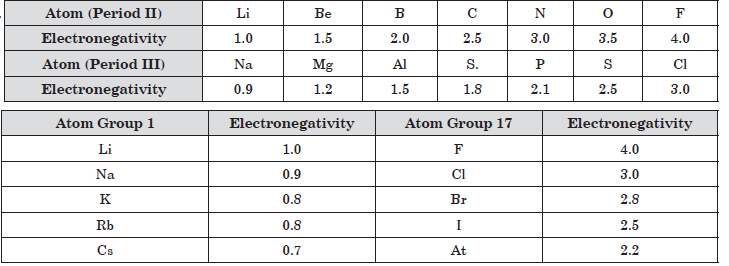
Question: How does electronegativity vary in a period?
a) Increases from left to right
b) Decreases from left to right
c) First increases then decreases
d) Vary independently
Answer: a
Question: What happens to the tendency to gain electrons in a period?
a) Increases
b) Decreases
c) Remains the same
d) First increases then decreases
Answer: a
Question: How does electronegativity vary in a group?
a) Increases down the group
b) Decreases down the group
c) First increases then decreases down the group
d) Vary independently
Answer: b
Question: Which element has the highest electronegativity?
a) C
b) N
c) O
d) F
Answer: d
Question: Which of the following has the least electronegativity?
a) Li
b) Be
c) O
d) N
Answer: a
Modern periodic table has 18 vertical columns known as groups and 7 horizontal rows known as periods. First period contains 2 elements second and third period contain 8 elements. 4th and 5th period contains 18 elements and 6th and 7th period contains 32 elements. The graph is plotted between atomic number and atomic radius of group 17 and group 1 elements.
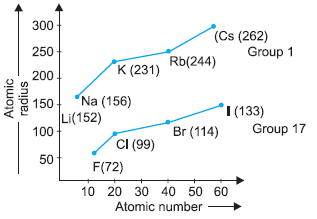
Question: Which group elements will gain electrons to form negative ions?
a) Group 1
b) Group 2
c) Group 17
d) Group 18
Answer: c
Question: Atomic size decreases from left to right in a period because:
a) Effective nuclear charge increases
b) Number of shells remains the same
c) Force of attraction between the nucleus and valence electrons increases
d) All of these
Answer: d
Question: Which element in Group 17 has the smallest size?
a) Fluorine
b) Bromine
c) Chlorine
d) Iodine
Answer: a
Question: Which group elements will have the largest atomic size?
a) Group 1
b) Group 2
c) Group 3
d) Group 18
Answer: a
Question: What happens to atomic radii in a group from top to bottom?
a) Increases
b) Decreases
c) First decreases then increases
d) Number of shells remains the same
Answer: a
Around the year 1800, only 30 elements were known. Dobereiner in 1817 and Newlands in 1866 tried to arrange the then known elements and framed laws which were rejected by the scientists. Even after the rejection of the proposed laws, many scientists continued to search for a pattern that correlated the properties of elements with their atomic masses.
The main credit for classifying elements goes to Mendeleev for his most important contribution to the early development of a Periodic table of elements wherein he arranged the elements on the basis of their fundamental property, the atomic mass and also on the similarity of chemical properties. The formulae of their hydrides and oxides were treated as basic criteria for the classification of the elements.
However, Mendeleev’s classification also had some limitations as it could not assign the position to isotopes. He also left some gaps in the periodic table.
Question: Why did Mendeleev leave some gaps in the Periodic Table?
a) For elements to be discovered
b) For isotopes
c) For isobars
d) None of these
Answer: a
Question: According to Mendeleev’s Periodic Law, properties of elements are periodic functions of:
a) Atomic mass
b) Atomic number
c) Number of protons
d) Number of electrons
Answer: a
Question: If the letter ‘R’ was used to represent any of the elements in the group, then the hydride and oxide of carbon would respectively be represented as:
a) RH₄, RO
b) RH₄, RO₂
c) RH₂, RO₂
d) RH₂, RO
Answer: b
Question: How many groups and periods are there in Mendeleev’s Periodic Table?
a) 6 groups, 8 periods
b) 18 groups, 7 periods
c) 7 groups, 18 periods
d) 8 groups, 6 periods
Answer: d
Question: Isotopes are:
a) Atoms of an element with similar chemical properties but different atomic masses
b) Atoms of different elements with similar chemical properties but different atomic masses
c) Atoms of an element with different chemical properties but same atomic masses
d) Atoms of different elements with different chemical properties but same atomic masses
Answer: a
Assertion-reason Type Questions :
For question numbers 1 to 2 two statements are given‑one labeled as Assertion (A) and the other labeled
Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both ‘A’ and ‘R’ are true and ‘R’ is correct explanation of the assertion.
(b) Both ‘A’ and ‘R’ are true but ‘R’ is not correct explanation of the assertion.
(c) ‘A’ is true but ‘R’ is false.
(d) ‘A’ is false but ‘R’ is true.
Question. Assertion: X with atomic number 13 is a metal.
Reason: It belongs to group 13 and 3rd period.
Answer: b
Question. Assertion: Carbon is a metalloid.
Reason: Carbon forms CO2 which is acidic oxide whereas CO is neutral oxide.
Answer: d
Important Practice Resources for Class 10 Science
MCQs for Chapter 5 Periodic Classification of Elements Science Class 10
Students can use these MCQs for Chapter 5 Periodic Classification of Elements to quickly test their knowledge of the chapter. These multiple-choice questions have been designed as per the latest syllabus for Class 10 Science released by CBSE. Our expert teachers suggest that you should practice daily and solving these objective questions of Chapter 5 Periodic Classification of Elements to understand the important concepts and better marks in your school tests.
Chapter 5 Periodic Classification of Elements NCERT Based Objective Questions
Our expert teachers have designed these Science MCQs based on the official NCERT book for Class 10. We have identified all questions from the most important topics that are always asked in exams. After solving these, please compare your choices with our provided answers. For better understanding of Chapter 5 Periodic Classification of Elements, you should also refer to our NCERT solutions for Class 10 Science created by our team.
Online Practice and Revision for Chapter 5 Periodic Classification of Elements Science
To prepare for your exams you should also take the Class 10 Science MCQ Test for this chapter on our website. This will help you improve your speed and accuracy and its also free for you. Regular revision of these Science topics will make you an expert in all important chapters of your course.
You can get most exhaustive CBSE Class 10 Chemistry Periodic Classification of Elements MCQs Set D for free on StudiesToday.com. These MCQs for Class 10 Science are updated for the 2025-26 academic session as per CBSE examination standards.
Yes, our CBSE Class 10 Chemistry Periodic Classification of Elements MCQs Set D include the latest type of questions, such as Assertion-Reasoning and Case-based MCQs. 50% of the CBSE paper is now competency-based.
By solving our CBSE Class 10 Chemistry Periodic Classification of Elements MCQs Set D, Class 10 students can improve their accuracy and speed which is important as objective questions provide a chance to secure 100% marks in the Science.
Yes, Science MCQs for Class 10 have answer key and brief explanations to help students understand logic behind the correct option as its important for 2026 competency-focused CBSE exams.
Yes, you can also access online interactive tests for CBSE Class 10 Chemistry Periodic Classification of Elements MCQs Set D on StudiesToday.com as they provide instant answers and score to help you track your progress in Science.

