Practice CBSE Class 10 Science Carbon and its Compounds MCQs Set E provided below. The MCQ Questions for Class 10 Chapter 4 Carbon and its Compounds Science with answers and follow the latest CBSE/ NCERT and KVS patterns. Refer to more Chapter-wise MCQs for CBSE Class 10 Science and also download more latest study material for all subjects
MCQ for Class 10 Science Chapter 4 Carbon and its Compounds
Class 10 Science students should review the 50 questions and answers to strengthen understanding of core concepts in Chapter 4 Carbon and its Compounds
Chapter 4 Carbon and its Compounds MCQ Questions Class 10 Science with Answers
Question: Antiknocking compound in gasoline is :
a) Triethyl lead
b) Trimethyl lead
c) Tetramethyl lead
d) Tetraethyl lead
Answer: d
Question: The substance that would not at all be formed during the reaction of methane and chlorine in presence of sunlight is
a) CH3Cl
b) CHCl3
c) CH3CH2CH3
d) CH2Cl2
Answer: c
Question: Which is not a property of graphite?
a) It melts at 800°C
b) It is a smooth, crystalline form of carbon
c) It is a good conductor of electricity
d) It forms a black sign on the paper
Answer: a
Question: Diamond is not a good conductor of electricity because
a) it is very hard
b) its structure is very compact
c) it is not water soluble
d) it has no free electrons
Answer: d
Question: A phenomenon by which an element occurs in different physical modification in same physical state is called
a) Isomerism
b) Allotropy
c) Amorphous
d) Crystalline
Answer: b
Question: Th e fo llowing reactio n is an example of CH4 + 2O2 → CO2 + 2H2O + heat + light
a) addition reaction
b) substitution reaction
c) combustion reaction
d) displacement reaction
Answer: c
Question: In order to form branching, an organic compound must have a minimum number of
a) four carbon atoms
b) three carbon atoms
c) five carbon atoms
d) six carbon atoms
Answer: a
Question: Which of the following is a pair of saturated hydrocarbon ?
a) Butane and isobutane
b) Cyclohexane and hexene
c) Propanal and propanone
d) All of the options
Answer: a
Question: Pentane has the molecular formula C5H12. It has
a) 5 covalent bonds
b) 12 covalent bonds
c) 16 covalent bonds
d) 17 covalent bonds
Answer: c
Question: Which of the following is purest form of carbon?
a) Diamond
b) Coal
c) Wood
d) Paper
Answer: a
Question: Which of the following does not contain double bond?
a) CO2
b) C2H4
c) HCl
d) O2
Answer: c
Question: The functional group present in butanone is
a) Carboxy
b) Ketonic
c) Aldehydic
d) Alcoholic
Answer: b
Question: Conversion of ethanol to ethanoic acid is a/an
a) substitution reaction
b) oxidation reaction
c) addition reaction
d) rearrangement reaction
Answer: a
Question: The IUPAC name of the compound CH2 = C(CH3)2 is
a) 1,1-Dimethylprop-2-ene
b) 2-Methylprop-1-ene
c) 2-Ethyl-3,3-dimethylbutane
d) 2,3-Dimethylhexane
Answer: b
Question: Methane, ethane and propane are said to form a homologous series because all are
a) hydrocarbons
b) saturated hydrocarbons
c) aliphatic hydrocarbons
d) differ from each other by –CH2 group
Answer: d
Question: Glacial acetic acid is
a) frozen acetic acid
b) 5–8% solution of acetic acid
c) mixture of acetic acid and alcohol
d) None of the options
Answer: b
Question: Ethene can be prepared by reaction of ethanol with
a) Hot conc. H2SO4
b) Alkaline KMnO4
c) Sodium metal
d) NaHCO3
Answer: a
Question: In fullerene carbon atoms are arranged in mixed
a) tetragons and pentagons
b) pentagons and hexagons
c) pentagons and heptagons
d) All are correct
Answer: b
Question: Soaps are sodium salts of fatty acids. Which of the following fatty acids does not form soap?
a) butyric acid
b) oleic acid
c) palmitic acid
d) stearic acid
Answer: a
Question: In shaving creams________ is added to prevent rapid drying.
a) Methanol
b) Glycerol
c) Ethanol
d) Glycol
Answer: b
Question: Nitrogen molecule involves formation of
a) Single covalent bond
b) Double covalent bond
c) Triple covalent bond
d) Ionic bond
Answer: c
Question: Which of the following statements is not correct ?
a) A common functional group is present in different members of a homologous series.
b) Two consecutive members of a homologous series differ by a –CH3 group.
c) The members of a homologous series can be represented by one general formula.
d) Different members of a homologous series have similar chemical properties.
Answer: b
Question: In the reaction CH3COONa + NaOH → the gas obtained is
a) C2H6
b) C2H2
c) CH4
d) C2H6
Answer: c
Question: Detergents can lather well in –
a) soft water
b) hard water
c) river water
d) any one of the above
Answer: d
Question: Unsaturation in the organic compound can be tested by the help of
a) Baeyer's test
b) Fehling's test
c) Chlorination reaction
d) Dehydration reaction
Answer: a
Question: When methane is burnt in an excess of air, the products of combustion are
a) C and H2O
b) CO and H2O
c) CO2 and H2
d) CO2 and H2O
Answer: d
Question: C2H4 reacts with hydrogen in presence of Ni to give
a) CH4
b) C2H6
c) HCOOH
d) HCHO
Answer: b
Question: Which of the following is not a saturated hydrocarbon?
a) Cyclohexane
b) Benzene
c) Butane
d) Isobutane
Answer: b
Question: The number of oxygen molecules used in the combustion of 1 molecule of ethanol is
a) 1
b) 2
c) 3
d) 4
Answer: c
Question: Two organic compounds ‘A’ and ‘B’ react with sodium metal and both produce the same gas ‘X’, but with sodium hydrogen carbonate, only compound B reacts to give a gas ‘Y’. Identify ‘A’, ‘B’, ‘X’ and ‘Y’:
a) A = Ethylene, B = Ethyl alcohol, X = Carbon dioxide, Y = Hydrogen
b) A = Ethyl alcohol, B = Acetic acid, X = Hydrogen, Y = Carbon dioxide
c) A = Methyl alcohol, B = Ethyl alcohol, X = Hydrogen, Y = Carbon dioxide
d) A = Acetic acid, B = Formic acid, X = Carbon dioxide, Y = Hydrogen
Answer: b
Question: Number of electrons shared between carbon carbon atoms in ethene is
a) 2
b) 4
c) 6
d) 8
Answer: b
Question: How many grams of oxygen gas will be needed for complete combustion of 2 moles of 3rd member of alkyne series ?
a) 186 g
b) 256 g
c) 352 g
d) 372 g
Answer: c
Question: In C6H14 the number of possible isomers is
a) 3
b) 6
c) 4
d) 5
Answer: a
Question: Ethanol on complete oxidation gives
a) carbon dioxide and water
b) acetaldehyde
c) acetic acid
d) acetone
Answer: c
Question: The by-product of soap industry is
a) glycerol
b) glycol
c) isoprene
d) acid
Answer: a
Question: Which of the following is not an allotropic form of carbon?
a) Fullerene
b) Fluorine
c) Diamond
d) Graphite
Answer: b
Question: The fermentation reactions are carried out in temperature range of –
a) 20-30°C
b) 30-40°C
c) 40-50°C
d) 50-60°C
Answer: a
Question: The difference in molecular weight of two consecutive members of a homologous series is
a) 15
b) 14
c) 8
d) 9
Answer: b
Question: The total number of electrons and the number of electrons involved in the formation of various bonds present in one molecule of propanal (C2H5CHO) are respectively.
a) 32 and 20
b) 24 and 20
c) 24 and 18
d) 32 and 18
Answer: a
Question: Among the following the one having longest chain is
a) Neopentane
b) Isopentane
c) 2-Methylpentane
d) 2,2-Dimethylbutane
Answer: c
Question: When ethanoic acid is heated with NaHCO3 the gas evolved is
a) H2
b) CO2
c) CH4
d) CO
Answer: b
Question: Which of the following gases is called 'Marsh gas'?
a) H2
b) CH4
c) C2H4
d) C2H2
Answer: b
Question: During laboratory preparation CH4 gas is collected by downward displacement of water because
a) CH4 is lighter than Air
b) CH4 is poisonous gas
c) It does not dissolve in water
d) All the above statements are correct
Answer: c
Question: The reaction hv 4 2 light 3 CH + Cl ¾¾¾→CH Cl +HCl is an example of
a) addition reaction
b) substitution reaction
c) elimination reaction
d) oxidation reaction
Answer: b
Question: How many unshared pairs of electrons are present in water molecule?
a) One
b) Zero
c) Two
d) Three
Answer: c
Question: Which of the following salts when dissolved in water produce hard water ?
a) Calcium sulphate
b) Magnesium bicarbonate
c) Calcium bicarbonate
d) All of the options
Answer: d
Question: The reaction, 2C2H5OH + 2Na → 2C2H5ONa + H2 suggests that ethanol is
a) acidic in nature
b) basic in nature
c) amphoteric in nature
d) neutral in nature
Answer: a
Question: A hydrocarbon ‘A’ (C3H8) on treatment with chlorine in presence of sunlight yielded compound ‘B’ as major product Reaction of ‘B’ with aqueous KOH gave ‘C’ which on treatment with concentrated H2SO4 yielded ‘D’. Hydrogenation of ‘D’ gave back ‘A’. The sequence of reactions involved in above conversion is:
a) substitution, substitution, addition, dehydration
b) substitution, substitution , dehydration, addition
c) substitution, dehydration, addition, addition
d) addition, substitution, dehydration, substitution.
Answer: b
Question: An organic liquid ‘A’ with acidified potassium dichromate gave product ‘B’. The compound ‘B’ on heating with methanol in presence of concentrated sulphuric acid formed compound ‘C’ which on subsequent treatment with sodium hydroxide formed two product ‘D’ and ‘E’. The product ‘D’ is known to affect the optic nerve causing blindness. Intake of ‘D’ in very small quantities can cause death. What are compound ‘A’, ‘B’, ‘C’, ‘D’ and ‘E’?
a) A = Ethanol, B = Ethanoic acid, C = Methanol D = Sodium acetate, E = Methyl ethanoate
b) A = Ethanol, B = Ethanoic acid, C = Methyl ethanoate D = Methanol, E = Sodium acetate
c) A = Sodium acetate, B = Ethanoic acid, C = Methyl ethanoate, D = Methanol, E = Ethanol
d) A = Ethanol, B = Ethanoic acid, C = Methyl ethanoate, D = Sodium acetate, E = Methanol
Answer: b
Question: The functional groups present in the following compound are –
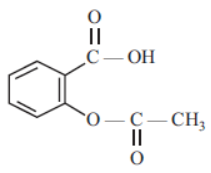
a) alcohol, ketone and ester
b) ester and carboxylic acid
c) carboxylic acid and ketone
d) ester and alcohol
Answer: b
Fill in the Blanks :
Question: The ability of carbon to form chains gives rise to a ............... series of compounds.
Answer: homologous
Question: Detergents cause ................. pollution.
Answer: water
Question: The soft crystalline form of carbon is ................ .
Answer: graphite
Question: Newly discovered allotrope of carbon is ............. .
Answer: fullerene
Question: Ethylene burns in air to form CO2 and ............... .
Answer: water
Question: Next homologue of ethane is ............... .
Answer: propane
Important Practice Resources for Class 10 Science
MCQs for Chapter 4 Carbon and its Compounds Science Class 10
Students can use these MCQs for Chapter 4 Carbon and its Compounds to quickly test their knowledge of the chapter. These multiple-choice questions have been designed as per the latest syllabus for Class 10 Science released by CBSE. Our expert teachers suggest that you should practice daily and solving these objective questions of Chapter 4 Carbon and its Compounds to understand the important concepts and better marks in your school tests.
Chapter 4 Carbon and its Compounds NCERT Based Objective Questions
Our expert teachers have designed these Science MCQs based on the official NCERT book for Class 10. We have identified all questions from the most important topics that are always asked in exams. After solving these, please compare your choices with our provided answers. For better understanding of Chapter 4 Carbon and its Compounds, you should also refer to our NCERT solutions for Class 10 Science created by our team.
Online Practice and Revision for Chapter 4 Carbon and its Compounds Science
To prepare for your exams you should also take the Class 10 Science MCQ Test for this chapter on our website. This will help you improve your speed and accuracy and its also free for you. Regular revision of these Science topics will make you an expert in all important chapters of your course.
You can get most exhaustive CBSE Class 10 Science Carbon and its Compounds MCQs Set E for free on StudiesToday.com. These MCQs for Class 10 Science are updated for the 2025-26 academic session as per CBSE examination standards.
Yes, our CBSE Class 10 Science Carbon and its Compounds MCQs Set E include the latest type of questions, such as Assertion-Reasoning and Case-based MCQs. 50% of the CBSE paper is now competency-based.
By solving our CBSE Class 10 Science Carbon and its Compounds MCQs Set E, Class 10 students can improve their accuracy and speed which is important as objective questions provide a chance to secure 100% marks in the Science.
Yes, Science MCQs for Class 10 have answer key and brief explanations to help students understand logic behind the correct option as its important for 2026 competency-focused CBSE exams.
Yes, you can also access online interactive tests for CBSE Class 10 Science Carbon and its Compounds MCQs Set E on StudiesToday.com as they provide instant answers and score to help you track your progress in Science.

