Read and download the CBSE Class 10 Science Carbon and its compound Sure Shot Questions A. Designed for 2025-26, this advanced study material provides Class 10 Science students with detailed revision notes, sure-shot questions, and detailed answers. Prepared by expert teachers and they follow the latest CBSE, NCERT, and KVS guidelines to ensure you get best scores.
Advanced Study Material for Class 10 Science Chapter 4 Carbon and Its Compound
To achieve a high score in Science, students must go beyond standard textbooks. This Class 10 Chapter 4 Carbon and Its Compound study material includes conceptual summaries and solved practice questions to improve you understanding.
Class 10 Science Chapter 4 Carbon and Its Compound Notes and Questions
CBSE Class 10 Science Chapter 4 Carbon and its compound MCQs
Question. Which of the following organic compounds does not have the same chemical properties as methanol?
(a) C2H6O
(b) C5H10O
(c) C4H10O
(d) C7H16O
Answer. B
Question. Which of the following is correct about the structure of diamond?
(a) Carbon atoms are held together by single covalent bonds.
(b) Electrons move freely through the structure.
(c) Layers of atoms slide easily over each other.
(d) Carbon atoms conduct electricity in the molten state.
Answer. A
Question. Z has the same boiling point as n-butane.
(a) 1, 2 and 3
(b) 2 and 4
(c) 1 and 4
(d) 1, 3 and 4
Answer. A
Question. How many structural isomers are possible for pentane (C5H12)?
(a) 1
(b) 4
(c) 2
(d) 3
Answer. D
Question. In C6H14 the number of possible isomers is
(a) 3
(b) 6
(c) 4
(d) 5
Answer. D
Question. __________ is widely used as a fuel and is a major component of bio-gas and CNG. It is also one of the simplest compounds formed by carbon.
(a) Ethane
(b) Propane
(c) Carbon dioxide
(d) Methane
Answer. D
Question. Which of the following compounds has a triple bond?
(a) C2H4
(b) C3H4
(c) C3H8
(d) C4H10
Answer. B
Question. Which of the following statements is correct?
(a) Most organic compounds are ionic compounds.
(b) Ethane and ethene belong to the same homologous series.
(c) Propene contains three hydrogen atoms per molecule.
(d) Chloroethane contains two carbon atoms per molecule.
Answer. D
Question. All the members of homologous series of alkynes have the general formula
(a) CnH2n
(b) CnH2n + 2
(c) CnH2n – 2
(d) CnH2n – 4
Answer. C
Question. Three hydrocarbons X, Y and Z are shown below :
X : CH3CH2CH2CH2CH3
Y : CH3 —C ≡C —CH2CH3
Z : CH3CH2— CH ≡CH —CH3
Identify the incorrect statements about these three hydrocarbons.
I. X and Y both differ by a CH2 unit.
II. X and Z have the same boiling point.
III. All have different general formulae.
IV. Y and Z have different molecular masses.
(a) I and II
(b) II and III
(c) I and IV
(d) All the statements are incorrect
Answer. A
Question. Which of the following statements is correct about a substance that has a giant covalent structure?
(a) All its ions are arranged in a giant three dimensional lattice.
(b) A large amount of energy is needed to break down the lattice structure.
(c) It conducts electricity in the molten state.
(d) It dissolves in water but is more soluble in organic solvents.
Answer. B
Question. The general formula of cycloalkanes is
(a) CnH2n + 2
(b) CnH2n – 2
(c) CnH2n – 1
(d) CnH2n
Answer. D
Question. Which of the following exists as a simple triatomic molecule?
(a) Argon
(b) Fluorine
(c) Nitrogen
(d) Ozone
Answer. D
Question. Which of the following molecules has all its atoms joined together by double covalent bonds?
(a) Methane
(b) Water
(c) Carbon dioxide
(d) Nitrogen trichloride
Answer. C
Question. Which of the following is not a characteristic of members of a homologous series?
(a) They possess varying chemical properties.
(b) Their physical properties vary in regular and predictable manner.
(c) Their formulae fit the general molecular formula.
(d) Adjacent members differ by one carbon and two hydrogen atoms.
Answer. A
Question. Which covalent molecule contains the structure where the central atom is bonded to four other atoms by covalent bonds?
1. Diamond 2. Graphite
3. Methane 4. Silicon dioxide
(a) 1 and 2
(b) 2 and 4
(c) 3 and 4
(d) 1, 3 and 4
Answer. D
Question. Which of the following represent saturated hydrocarbons?
1. CH(CH3)3
2. CH2CHCH3
3. CH3CH(CH3)CH2CH3
4. (CH3)2CCHCH3
(a) 1 and 3
(b) 2 and 4
(c) 1, 2 and 3
(d) 1, 2, 3 and 4
Answer. A
Question. Which of the following has shortest carboncarbon bond length?
(a) C2H2
(b) C2H4
(c) C2H6
(d) C6H6
Answer. A
Question. Which type of bond is present between carbon-carbon atoms in acetylene?
(a) Single covalent bond
(b) Double covalent bond
(c) Triple covalent bond
(d) Electrovalent bond
Answer. C
Question. Number of free electron(s) in each carbon atom in graphite is/are
(a) two
(b) four
(c) one
(d) three.
Answer. C
Question. Which of the following statements is not correct?
(a) A common functional group is present in different members of a homologous series.
(b) Two consecutive members of a homologous series differ by a –CH3 group.
(c) The members of a homologous series can be represented by one general formula.
(d) Different members of a homologous series have similar chemical properties.
Answer. B
Question. Identify the correct statements.
(i) As the molecular mass increases in any homologous series, a gradation in physical properties is seen.
(ii) The melting and boiling points decrease with increasing molecular mass.
(iii) Other physical properties such as solubility in a particular solvent decreases with increasing molecular mass.
(iv) The chemical properties, which are determined solely by the functional group, remain similar in a homologous series.
(a) (ii) and (iii)
(b) (ii) and (iv)
(c) (i), (iii) and (iv)
(d) All are correct
Answer. C
CBSE Class 10 Science Chapter 4 Carbon and its compound Assertion and Reason
For question numbers 56-70, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Both assertion and reason are true, and reason is correct explanation of the assertion.
(b) Both assertion and reason are true, but reason is not the correct explanation of the assertion.
(c) Assertion is true, but reason is false.
(d) Assertion is false, but reason is true.
Question. Assertion : In alkanes, alkenes and alkynes the valency of carbon is always four.
Reason : All hydrocarbons except alkanes contain double bonds.
Answer. C
Question. Assertion : Carbon possesses property of catenation.
Reason : Carbon atoms form double as well as triple bonds during catenation.
Answer. B
Question. Assertion : Ethanol is first member of the alcohol homologous series.
Reason : A homologous series can be represented by a general formula.
Answer. D
Question. Assertion : Diamond is the hardest natural known substance.
Reason : Diamond is used for cutting marble, granite and glass.
Answer. B
Question. Assertion : Diamond is not a good conductor of electricity.
Reason : It has no free electrons.
Answer. A
Question. Assertion : Two members of a homologous series have similar chemical properties.
Reason : Propane and butane are members of same homologous series.
Answer. B
Question. Assertion : Saturated hydrocarbons are chemically less reactive.
Reason : All the valencies of carbon atom are satisfied by single covalent bonds.
Answer. A
Question. Assertion : Covalent compounds are generally poor conductor of electricity.
Reason : They consist of molecules and not ions which can transfer charge.
Answer. A
Question. Assertion : Graphite is a good conductor of electricity.
Reason : It has one free valence electron.
Answer. A
Question. Assertion : The functional group present in alcohols is –OH.
Reason : It is the same group as present in water, hence water and alcohol have similar properties.
Answer. C
Question. Assertion : Diamond and graphite do not have the same crystal structure.
Reason : Diamond is crystalline while graphite is amorphous.
Answer. C
Question. Assertion : Carbon and its compounds can be used as fuels.
Reason : They are highly inflammable and have high calorific value.
Answer. A
Question. Assertion : Olefins have the general formula CnH2n+1.
Reason : There is atleast one double bond between two carbon atoms in their molecules.
Answer. D
Question. Assertion : Graphite is soft and slippery to touch.
Reason : Graphite has sheet like layered structure.
Answer. A
Question. Assertion : Both aldehydes and ketones contain carbonyl group.
Reason : In aldehydes, the functional group is attached to atleast one hydrogen atom.
Answer. B
CBSE Class 10 Science Chapter 4 Carbon and its compound Case Based MCQs
Case I : Read the passage given below and answer the following questions.
An organic molecule has the following structure :
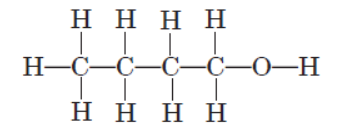
Question. To which homologous series does this molecule belong?
(a) Aldehydes
(b) Ketones
(c) Alcohols
(d) Alkanes
Answer. C
Question. What is the general formula of this homologous series?
(a) CnH2n + 1OH
(b) CnH2n + 2
(c) CnH2nO
(d) CnH2n + 1CHO
Answer. A
Question. Which is the next member of this series?
(a) C4H9OH
(b) C3H7OH
(c) C5H11OH
(d) C6H13OH
Answer. C
Question. Which is the third member of this series?
(a) C3H7OH
(b) C4H9OH
(c) C2H5OH
(d) CH3OH
Answer. A
Question. Which is the second member of this series?
(a) Ethanol
(b) Methanol
(c) Propanol
(d) Butanol
Answer. A
Case II : Read the passage given below and answer the following questions.
When an element exists in two or more different forms in the same physical state, these different forms are called allotropes and the phenomenon is known as allotropy. Allotropes have similar chemical properties but they differ in their physical properties. Carbon exists in crystalline and amorphous forms. In crystalline form, it occurs as diamond, graphite and fullerenes. Diamond is a colourless, transparent substance having extraordinary brilliance. It is the hardest natural substance known. It is used for cutting marble, granite and glass. Graphite is a greyishblack, opaque substance. It is lighter than diamond i.e., it has lower density. It has sheet like structure having hexagonal layers. One layer slides over the other layer which makes it soft to touch. It is the reason that graphite is used as a lubricant.
Question. Substance X is a moderate conductor of electricity. Substance X has the structure shown below :
Which statements about substance X are correct?
(I) It is a covalent compound.
(II) It has a giant molecular structure.
(III)It has the same structure as graphite.
(IV) It has the same structure as diamond.
(a) (I) and (III)
(b) (II) and (III)
(c) (II) and (IV)
(d) (I), (II) and (IV)
Answer. C
Question. Which of the following is correct about the structure of diamond?
(a) Carbon atoms are held together by single covalent bonds.
(b) Electrons move freely through the structure.
(c) Layers of atoms slide easily over each other.
(d) Carbon atoms conduct electricity in the molten state.
Answer. A
Question. Which three allotropes of carbon, do the given figures represent?
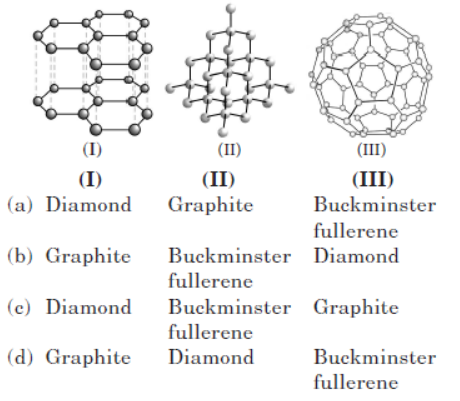
Answer. D
Question. Identify the incorrect statement(s).
(I) Diamond is the hardest substance known while graphite is smooth and slippery.
(II) Diamond is made up of billions of carbon atoms. Each carbon atom is bonded to four other carbon atoms in a tetrahedral manner to form a giant lattice. All carbon atoms are bonded by strong covalent bonds.
(III) Graphite is a poor conductor of electricity unlike other non-metals.
(IV) Graphite has a giant covalent structure that is made up of layers of carbon atoms.
In each layer, each carbon atom is bonded to three other carbon atoms to form hexagonal rings of carbon atoms.
(a) (I) and (III)
(b) Only (III)
(c) (II) and (IV)
(d) (I), (II) and (IV)
Answer. B
Question. Structures of two different forms of carbon are given below :
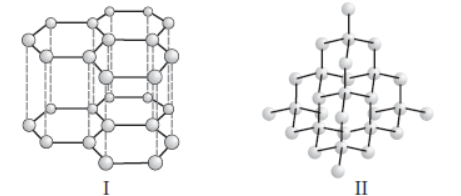
Identify the two forms (I and II respectively) and how are they related to each other?
(a) Diamond, Graphite, Isotopes of carbon
(b) Graphite, Diamond, Allotropes of carbon
(c) C12, C14, Allotropes of carbon
(d) C14, C12, Isotopes of carbon
Answer. B
CARBON AND ITS COMPOUND
1. Which of the following is not a saturated hydrocarbon ?
i) Cyclohexane.
ii) Benzene.
iii) Butane
iv) isobutene
2. The bond between two identical non metallic atom has a pair of electron ?
i) un equally shared between two atoms.
ii) Transferred completely from one atom to another.
iii) With identical spins
iv) Unequally shared between them.
3. Covalent compounds are generally
i) Soluble in water
ii) Insoluble in water
iii) Ionize in water
iv) Hydrolyse in water
4. Propane with the molecular formula C3H8 has
i) 7covalent bonds
ii) 8 covalent bonds
iii) 9 covalent bonds
iv) 10 Covalent bonds.
5. A hydrocarbon reacts with ammonical cuprous chloride solution to form a red precipitate .The hydrocarbon is
i)ethane
ii)ethene
iii)butane
iv)1-propyne
6. Which of the following substance is added to denature Ethanol ?
i)methanol ii)pyridine
iii)copper sulphate iv)all of them
7. Which of the following is not an allotropic form of carbon
i)fluorine ii)fullerene
iii)diamond iv)graphite
8. Which of the following represents the correct decreasing order of hydrogen atoms ?
i)alkanes , alkenes , alkynes
ii)alkanes , alkaynes , alkenes
iii)alkenes , alkynes , alkanes
iv)alkynes , alkanes , alkenes
9. Detergents are sodium or potassium salts of long chain of ;-
i)aldehydes
ii)ketones
iii)carboxylic acid
iv)sulphonic acid
10. Which of the following represents the structure of N2 Mmolecule ?
i) N≡ N
ii) N = N
iii) N - N
iv) None of the above
11. In double covalent bond there is sharing of
i) 2 electrons
ii) 4 electrons
iii) 6 electrons
iv) 3 electrons
12. Cation is formed when
i) atom gains electrons
ii) atom loses electrons
iii) proton is lost by the atom
iv) atom shared by electrons
13. The total no. of electrons that take part in forming a bond in N2 is
i) 2
ii) 4
iii) 6
iv) 10
14. Which of the following has the weakest carbon-carbon strength?
i)C2H2
ii)C2H4
iii)C2H6
iv)all have the same bond strength
15. Which of the following salt when dissolved in water produce hard water.
i) calcium sulphate
ii) magnesium bicarbonate
iii) calcium chloride
iv) any of the above
16. Which of the following is not a saturated hydrocarbon ?
i) cyclohexane
ii) benzene
iii) butane
iv) isobutane
17. The bond between two identical nonmetallic atom has a pair of electron ?
i) unequally shared between two atoms
ii) transferred completely from one atom to another
iii) With identical spins
iv) Equally shared between them
18. Covalent compounds are generally –
i) Soluble in water
ii) insoluble in water
iii) Ionize in water
iv) hydrolyse in water
19. Propane with molecular formula C3H8 has –
i) 7 covalent bonds
ii) 8 covalent bonds
iii) 9 covalent bonds
iv) 10 covalent bonds
20. A hydrocarbon reacts with ammonical cuprous chloride solution to form a red precipitate,the hydrocarbon is –
i) Ethane
ii) ethane
iii) butane
iv) 1-propyne
21. Which of the following substance is added to denature Ethanol?
i) Methanol ii) pyridine
iii) copper sulphate iv) all of these
22. Which of the following is not an allotropic form of carbon ?
i) fluorine
ii) fullerene
iii) diamond
iv) graphite
23. Which of the following represents the correct deceasing order of hydrogen atoms ?
i) alkanes, alkenes, alkynes
ii) alkanes, alkynes, alkenes
iii) alkenes, alkynes, alkanes
iv) alkynes, alkanes, alkenes
24. Detergents are sodium or potassium salts of long chain of :
i) aldehydes
ii) ketones
iii) carboxylic acid
iv) sulphonic acid
25. In double covalent bond there is a sharing of
i) 2 electrons
ii) 4 electrons
iii) 6 electrons
iv) 3 electrons
26. Cation is formed when
i) atom gains electrons
ii) atom losses electrons
iii) proton is lost by the atom
iv) atom shared by electrons
27. The total number of electrons that take part in forming a bond in N2 is
i) 2
ii) 4
iii) 6
iv) 10
28. Which of the following has the weakest carbon-carbon strength ?
i) C2H2
ii) C2H4
iii) C2H6
iv) all have the same bond strength
29. Which of the following salt when dissolved in water produce hard water ?
i) calcium sulphate
ii) magnesium bicarbonate
iii) calcium chloride
iv) any of the above.
30. The two colours seen at the extreme ends of the pH charts are:-
i) red and blue
ii)red and green
iii) green and blue
iv) orange and green
31. Carboxylic acids on heating with P2O5 gives:-
i) ethers
ii) alcohol
iii) carbonyl compounds
iv) anhydrides
32. Synthetic flavours contain:-
i) unsaturated acids
ii) esters
iii) dilute carboxylic acids
iv) hydroxyl acids
33. Out of the following which one is used as preservative for pickle and sauces:-
i) esters ii) acetone
iii) aldehyde iv) acetic acid
34. Brisk effervescences produced when a pinch of Na2CO3 is added to CH3COOH is due to
the formation of :-
i) H2 gas ii) CO2 gas
iii) CO gas iv) CH4 gas
35. When an acetic acid reacts with an alcohol in the presence of conc. H2SO4:-
i) esters are formed ii) ketones are formed
iii) aldehydes are formed iv) none of these
36. Sodium bi carbonate solution is added to dilute Ethanoic acid. It is observed that:-
i) a gas evolves
ii) a solid settles at the bottom
iii) the mixture becomes vapour
iv) the colour of the mixture becomes light Yellow
37. Ethanoic acid was added to sodium bicarbonate sol. And the gas evolved was tested with a urning splinter. The following four observations were reported:-
1) the gas burns with the pop sound and the flame gets extinguished.
2) the gas does not burn out but the splinter burns with a pop sound
3) the flame extinguishes and the gas does not burn
4) the gas burns with a blue flame and the splinter burns brightly.
The correct observation is reported in:-
i) 1
ii) 2
iii) 3
iv) 4
38. 2ml of ethanoic acid was taken in each test tube 1 and 2 .A red litmus paper was introduced in test tube 1 and a pH paper was introduced in test tube 2. The experiment was performed by 4 students A, B, C, D and they reported their observation as given in the table. Student action on red action on litmus PH paper
A) Turned blue turned pink
B) Remains unchanged turned green
C) Turned blue turned blue
D) Remains unchanged turned pink
The correct observation is reported in
i) A
ii) B
iii) C
iv) D
39. Acetic acid was added to a solid X kept in a Test tube. A colourless, odourless gas Y was evolved. The gas was passed through the lime water, which turned milky. It concludes that:-
i) solid X is NaOH and the gas Y is CO2
ii) solid X is Na2CO3 and the gas Y is CO2
iii) solid X is sodium acetate and the gas y is CO2
iv) solid X is sodium chloride and the gas Y is CO2
40. Why is carbon tetravalent?
41. The formula of a hydrocarbon is CnH2n. Name the family to which it belongs and also predicts its nature.
42. What is the valency of carbon in CH3-CH3, CH2=CH2 and HC=CH ?
43. Out of butter and ground nut oil , which is unsaturated in Nature?
44. Why is high temperature not favourable for alcoholic fermentation?
45. Name a cyclic unsaturated hydrocarbon, containing three double bonds?
46. What is the difference in the molecular mass of any two adjacent homologues?
47. Which has triple bond ; C2H4 ,C3H6 and C3H4 ?
48. Which substance is added to denature ethyl alcohol?
49. Which ions are responsible for making water hard?
50. Name the catalyst commonly used in hydrogenation of oil to form fats?
51. Write the name and molecular formula of alcohol derived from butane ?
52. Which gas is evolved when sodium carbonate or bicarbonate is added to ethanoic acid?
53. What is SCUM ?
54. What are hydrophobic and hydrophilic parts in soaps?
55. How much percentage of earth’s crust constitutes carbon element ?
56. What do you mean by covalency ?
57. What is covalent bond ?
58. What is functional group ?
59. What is organic chemistry ?
60. What name is given to the reaction which take place when Ethanoic acid reacts with ethanol in the presence of conc. Sulphuric acid ? Name the products obtained in this reaction.
61. What is bromination ? Write the structural formula of product obtained on bromination of propene.
62. Define covalency ?
63. Write the structural formula of the isomers of n-butane?
64. Name the organic acid present in vinegar. Write its Chemical formula also.
65. The structural formula of an ester is HCOOCH2CH2CH3 write the formula of acid and the alcohol from which it is made ?
66. What happens when ethanol reacts with
(i) sodium
(ii) potassium permanganate solution.
67. Which of the following hydrocarbons undergo addition reactions : C2H6, C3H8, C3H6, C2H2 and CH4.
68. What is hydrogenation? Write its industrial application.
69. Give a test that can be used to differentiate between butter and cooking oil ?
70. Give the names of the functional group;-
Please click the link below to download CBSE Class 10 Science Carbon and its compound Sure Shot Questions A.
| CBSE Class 10 Science Electricity |
| CBSE Class 10 Science Electricity Notes |
| CBSE Class 10 Science Electricity Sure Shot Questions A |
| Class 10 Science Electricity Exam Notes |
Important Practice Resources for Free Printable Worksheets PDF
CBSE Class 10 Science Chapter 4 Carbon and Its Compound Study Material
Students can find all the important study material for Chapter 4 Carbon and Its Compound on this page. This collection includes detailed notes, Mind Maps for quick revision, and Sure Shot Questions that will come in your CBSE exams. This material has been strictly prepared on the latest 2026 syllabus for Class 10 Science. Our expert teachers always suggest you to use these tools daily to make your learning easier and faster.
Chapter 4 Carbon and Its Compound Expert Notes & Solved Exam Questions
Our teachers have used the latest official NCERT book for Class 10 Science to prepare these study material. We have included previous year examination questions and also step-by-step solutions to help you understand the marking scheme too. After reading the above chapter notes and solved questions also solve the practice problems and then compare your work with our NCERT solutions for Class 10 Science.
Complete Revision for Science
To get the best marks in your Class 10 exams you should use Science Sample Papers along with these chapter notes. Daily practicing with our online MCQ Tests for Chapter 4 Carbon and Its Compound will also help you improve your speed and accuracy. All the study material provided on studiestoday.com is free and updated regularly to help Class 10 students stay ahead in their studies and feel confident during their school tests.
Our advanced study package for Chapter Chapter 4 Carbon and Its Compound includes detailed concepts, diagrams, Mind Maps, and explanation of complex topics to ensure Class 10 students learn as per syllabus for 2026 exams.
The Mind Maps provided for Chapter Chapter 4 Carbon and Its Compound act as visual anchors which will help faster recall during high-pressure exams.
Yes, teachers use our Class 10 Science resources for lesson planning as they are in simple language and have lot of solved examples.
Yes, You can download the complete, mobile-friendly PDF of the Science Chapter Chapter 4 Carbon and Its Compound advanced resources for free.
Yes, our subject matter experts have updated the Chapter Chapter 4 Carbon and Its Compound material to align with the rationalized NCERT textbooks and have removed deleted topics and added new competency-based questions.

