Download the latest CBSE Class 11 Chemistry Redox Reactions Notes Set E in PDF format. These Class 11 Chemistry revision notes are carefully designed by expert teachers to align with the 2025-26 syllabus. These notes are great daily learning and last minute exam preparation and they simplify complex topics and highlight important definitions for Class 11 students.
Chapter-wise Revision Notes for Class 11 Chemistry Chapter 7 Redox Reactions
To secure a higher rank, students should use these Class 11 Chemistry Chapter 7 Redox Reactions notes for quick learning of important concepts. These exam-oriented summaries focus on difficult topics and high-weightage sections helpful in school tests and final examinations.
Chapter 7 Redox Reactions Revision Notes for Class 11 Chemistry
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron in a covalent bond belongs entirely to more electronegative element.
Calculation of oxidation number-
1. O. S. of all the elements in their elemental form (in standard state) is taken as zero O. S. of elements in Cl2, F2, O2, P4, O3, Fe(s), H2, N2, C(graphite) is zero.
2. Common O. S. of elements of group one (1st) is one. Common O. S. of elements of group two (2nd) is two.
3. For ions composed of only one atom, the oxidation number is equal to the charge on the ion.
4. The oxidation number of oxygen in most compounds is –2 .While in peroxides (e.g., H2O2, Na2O2), each oxygen atom is assigned an oxidation number of –1, in super oxides (e.g., KO2,Rb O2) each oxygen atom is assigned an oxidation number of – (½).
5. In oxygen difluoride (OF2) and dioxygen difluoride (O2F2), the oxygen is assigned an oxidation number of +2 and +1,respectively.
6. The oxidation number of hydrogen is +1 but in metal hydride its oxidation no. is–1.
7. In all its compounds, fluorine has an oxidation number of –1.
8. The algebraic sum of the oxidation number of all the atoms in a compound must be zero.
9. In polyatomic ion, the algebraic sum of all the oxidation numbers of atoms of the ion must equal the charge on the ion.
Stock notation: the oxidation number is expressed by putting a Roman numeral representing the oxidation number in parenthesis after the symbol of the metal in the molecular formula. Thus aurous chloride and auric chloride are written as Au(I)Cl and Au (III) Cl3. Similarly, stannous chloride and stannic chloride are written as Sn(II)Cl2andSn(IV)Cl4.
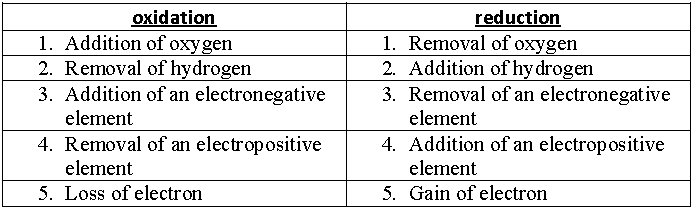
Oxidation : An increase in the oxidation number
Reduction : A decrease in the oxidation number
Oxidising agent : A reagent which can increase the oxidation number of an element in a given substance. These reagents are called as oxidants also.
Reducing agent : A reagent which lowers the oxidation number of an element in a given substance. These reagents are also called as reductants.
Redox reactions: Reactions which involve change in oxidation number of the interacting species
Balancing of redox reactions:
Oxidation Number Method:
Write the net ionic equation for the reaction of potassium dichromate(VI), K2Cr2O7 with sodium sulphite,Na2SO3, in an acid solution to give chromium (III) ion and the sulphate ion.
Step 1: The skeletal ionic equation is:
Cr2O72–(aq) + SO32–(aq) → Cr3+(aq)+ SO42–(aq)
Step 2: Assign oxidation numbers forCr and S
+6 –2 +4 –2 +3 +6 –2
Cr2O72–(aq) + SO32–(aq) → Cr3+(aq)+ SO42–(aq)
Step 3: Calculate the increase and decrease of oxidation number, and make them equal:
+6 –2 +4 –2 +3 +6
Cr2O72–(aq) + 3SO32–(aq) → 2Cr3+(aq)+ 3SO42–(aq)
Step 4: Balance the charge by adding H+ as the reaction occurs in the acidic
medium,
Cr2O72–(aq) + 3SO32–(aq) 8H+→ 2Cr3+(aq)+ 3SO42–(aq)
Step 5: Balance the oxygen atom by adding water molecule.
Cr2O72–(aq) + 3SO32–(aq) 8H+→ 2Cr3+(aq)+ 3SO42–(aq)+ 4H2O (l)
Half Reaction Method
balance the equation showing the oxidation of Fe2+ ions to Fe3+ ions by dichromate ions (Cr2O7)2– in acidic medium, wherein, Cr2O72– ions are reduced to Cr3+ ions.
Step 1 : Produce unbalanced equation for the reaction in ionic form :
Fe2+(aq) + Cr2O72– (aq) → Fe3+ (aq) + Cr3+(aq)
Step 2 : Separate the equation into half reactions:
+2 +3
Oxidation half : Fe2+ (aq) → Fe3+(aq)
+6 –2 +3
Reduction half :Cr2O72–(aq) → Cr3+(aq)
Step 3 : Balance the atoms other than O and H in each half reaction individually.
Cr2O72– (aq) → Cr3+(aq)
Step 4 : For reactions occurring in acidic medium, add H2O to balance O atoms and H+ to balance H atoms.Cr2O7
2– (aq) +14 H+→ Cr3+(aq) + 7H2O (l)
Step 5 : Add electrons to one side of the half reaction to balance the charges. If need be ,make the number of electrons equal in the two half reactions by multiplying one or both half reactions by appropriate coefficients.
Fe2+ (aq) → Fe3+ (aq) + e– Cr2O72– (aq) + 14H+ (aq) + 6e– → 2Cr3+(aq) +7H2O (l)
6Fe2+ (aq) →6 Fe3+ (aq) +6 e–
Step 6 : We add the two half reactions to achieve the overall reaction and cancel the electrons on each side. This gives the net ionic equation as :
6Fe2+(aq) + Cr2O72– (aq) + 14H+(aq) → 6 Fe3+(aq) + 2Cr3+(aq) + 7H2O (l)
A redox couple is defined as having together the oxidised and reduced forms of a substance taking part in an oxidation or reduction half reaction.
Represented as Zn2+/Zn and Cu2+/Cu.
- Electrochemical cells are the devices which are used to get electric current by using chemical reaction.
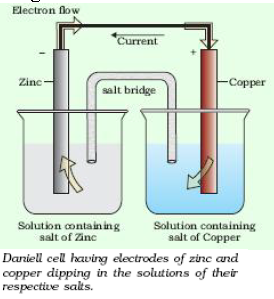
The potential associated with each electrode is known as electrode potential. If the concentration of each species taking part in the electrode reaction is unity (if any gas appears in the electrode reaction, it is confined to 1 atmospheric pressure) and further the reaction is carried out at 298K, then the potential of each electrode is said to be the Standard Electrode Potential.
- SHE is used to measure electrode potential and its standard electrode potential is taken as 0.00 V.
Question. Define oxidation and reduction in terms of oxidation number.
Answer: Increase in Oxidation Number is Oxidation and decrease in Oxidation Number is called reduction.
Question. What is meant by disproportionation? Give one example.
Answer: In a disproportionation reaction an element simultaneously oxidized and reduced.
P4 + 3OH– +3H2O → PH3 +3H2PO2-
Question. What is O.N. of sulphur in H2SO4?
Answer: +6
Question. Identify the central atom in the following and predict their O.S. HNO3
Answer: central atom:- N; O.S. +5
Question. Out of Zn and Cu which is more reactive?
Answer: Zn.
Question. What is galvanization?
Answer: Coating of a less reactive metal with a more reactive metal e.g.- coating of iron surface with Zn to prevent rusting of iron.
Question. How is standard cell potential calculated using standard electrode potential?
Answer: E0 cell = E0 cathode – E0 anode
Question. What is O.S. of oxygen in H2O2?
Answer: – -1.
Question. The formation of sodium chloride from gaseous sodium and gaseous chloride is a redox process justify.
Answer: Na atom get oxidize and Cl is reduced.
Question. Write the balanced redox reaction .
(I) MnO4 –(aq) + Fe2+(aq) → Mn2+(aq)+ Fe3+(aq) [acidic medium]
(II) Cr2O72– + Fe2+ →Cr3+ + Fe3+ [Acidic medium]
Answer: (i) MnO4–(aq) +5Fe2+(aq) + 8H+(aq) → Mn2+(aq)+ 5Fe3+(aq) + 4H2O(l)
(ii) Cr2O72– +6Fe2+ + 14H+→ 2Cr3+ + 6Fe3+ + 7H2O
Question. Identify the strongest & weakest reducing agent from the following metals: .Zn, Cu, Na, Ag, Sn
Answer: Strongest reducing agent: Na, weakest reducing agent: Ag.
Question. Determine the oxidation no. of all the atoms in the following oxidants:KMnO4,K2Cr2O7 and KClO4
Answer: In KMnO4 K = +1, Mn = +7, O = -2
In K2Cr2O7K = +1, Cr = +6, O = -2
In KClO4K = +1, Cl = =+7, O = -2
Question. Determine the oxidation no. of all the atoms in the following species:Na2O2 and OF2.
Answer: In Na2O2Na = +1, O = -1
InOF2, F = -1, O = +2
Question. Is it possible to store :
(i) H2SO4 in Al container?
(ii) CuSO4 solution in Zn vessel?
Answer: (i) yes. (ii) No.
Question. Calculate the standard e.m.f. of the cell formed by the combination of Zn/Zn2+⎤⎤ Cu2+/Cu.
Answer: E0
cell = E0 cathode – E0 anode
=0.34 – (-0.76) = 1.10V.
Question. Identify the oxidizing and reducing agents in the following equations:
(i) MnO4–(aq) +5Fe2+(aq) + 8H+(aq) → Mn2+(aq)+ 5Fe3+(aq) + 4H2O(l)
(ii) Cr2O72– +6Fe2+ + 14H+→ 2Cr3+ + 6Fe3+ + 7H2O
Answer: (i) O.A. = MnO4– ; R.A.= Fe2+
(ii)O.A.=Cr2O72–; R.A.= Fe2+
Question. Predict all the possible oxidation states of Cl in its compounds.
Answer: – 0, -1, +1, +3, +5, +7
Question. Formulate possible compounds of „Cl‟ in its O.S.is: 0, -1, +1, +3, +5, +7
Answer: Cl2, HCl, HOCl, HOClO, HOClO2, HOClO3 respectively.
Question. List three measures used to prevent rusting of iron.
Answer: (i) galvanization(coating iron by a more reactive metal)
(ii) greasing/oiling
(iii) painting.
Question. Write short notes on :
(a) Electrochemical series(b) redox reactions (c) oxidizing agents
Answer: (a) Electrochemical series :- arrangement of metals(non-metals also) in increasing order of their reducing power or vice versa.
(b) Reactions in which both Oxidation and reduction take place simultaneously are REDOX REACTIONS.
(c)oxidizing agents : chemical specie which can oxidize the other one or can reduce itself.
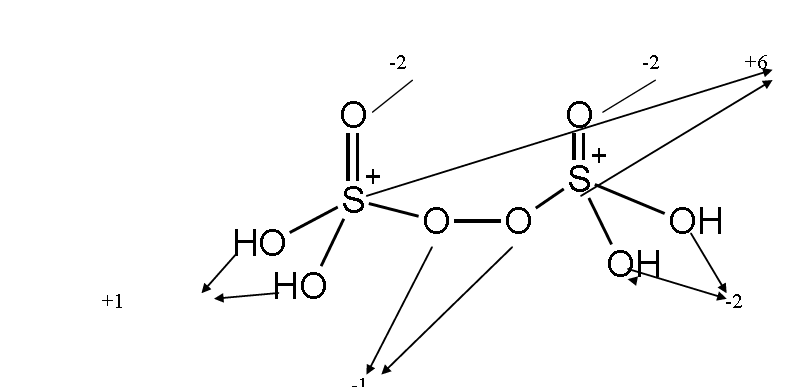
Question. Calculate O. S. of sulphur in the following oxoacids of „S‟ : H2SO4 ,H2SO3H2S2O8and H2S2O7
Answer: +6, +4, + 6 and +6 respectively. (calculate by considering x of ‟S‟ and taking +1 of H, -2 0f “O” and -1 of “O” in peroxide bond.)
Question. Explain role of salt bridge in Daniell cell.
Answer: (a) it completes the electric circuit in the cell.
(b) it maintains the electric neutrality in the cell.
Question. Account for the followings :
(i) sulphur exhibits variable oxidation states.
Answer: Due to the presence of vacant „d‟ orbitals in „S‟
(ii) Fluorine exhibits only -1 O.S.
Answer: It is most electronegative element
(iii) oxygen can’t extend its valency from 2.
Answer: Small size/unavailability of vacant „d‟ orbitals in O
Question. Balance the equation MnO4– + I– → Mn2+ +I2 + H2O by ion electron method in acidic medium.
Answer: Step -I Balancing of reduction half reaction by adding protons and electrons on LHS and more water molecules on RHS: 8H++ MnO4 – +5e-→ Mn2+ + 4H2O
Step – II Balancing of oxidation half reaction by adding electrons on RHS: 2I–→ I2 +2e-
Step – III To multiply the OHR by 5; RHR by2 and to add OH & RH reactions to get overall redox reaction(cancellation of electrons of RH & OH reactions):
[8H+(aq)+ MnO4 –(aq) +5e-→ Mn2+(aq) + 4H2O (l)] x 2 [ 2I-→ I2 +2e-] x 5 MnO4
– (aq) +5Fe2+(aq) + 8H+(aq) → Mn2+(aq)+ 5Fe3+(aq) + 4H2O(l)
Question. complete and balance the following equations:
(i) H+ + Cr2O7
2–+ Br–→ 2Cr3+ + Br2+ —–
(ii) H2O2 + Cl–→ OH– + Cl2
(iii) Zn + Cu2+ → ?
Answer: (i) 14H+ + Cr2O7
2–+6 Br–→ 2Cr3+ + 3Br2 + 7H2O
(ii) H2O2 + 2Cl–→ 2OH– + Cl2
(ii) Zn + Cu2+ → Zn2+ + Cu
Question. Identify the oxidizing and reducing agents in the following equations:
(i) Fe + H2SO4 → FeSO4 + H2
(ii)H2 + Cl2 →2HCl
(iii) MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
Answer: (i) O.A. = H2SO4 ; R.A.= Fe
(ii) O.A. = Cl2; R.A.=H2
(iii) O.A. = MnO2; R.A. =HCl
question. Arrange the following in increasing order of their reducing power:
Cu, Ag, Au, Zn, Fe, Al, Na, Mg, Pt(SHE), Hg, Ca, K
Answer: Au, Hg, Ag, Cu, Pt(SHE), Fe, Zn, Al, Mg, Na, Ca, K
Question. Indicate O.S. of each atom present in given structure of peroxodisulphuric acid
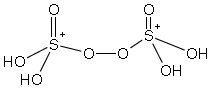
Answer:
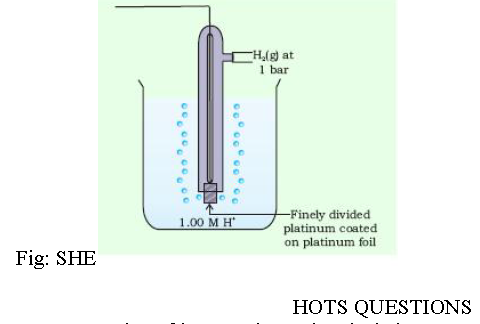
Question. What is SHE? What is its use?
Answer: Standard Hydrogen Electrode (SHE) has been selected to have zero standard potential at all temperatures. It consists of a platinum foil coated with platinum black (finely divided platinum) dipping partially into an aqueous solution in which the activity (approximate concentration 1M) of hydrogen ion is unity and hydrogen gas is bubbled through the solution at 1 bar pressure. The potential of the other half cell is measured by constructing a cell in which reference electrode is standard hydrogen electrode. The potential of the other half cell is equal to the potential of the cell.
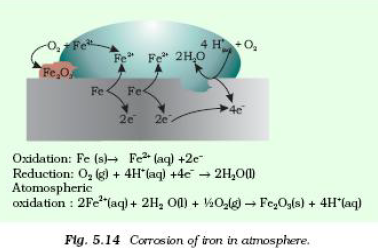
Question. Is rusting of iron an electrochemical phenomenon? How ?explain.
Answer: Yes. Rusting of iron is an electrochemical phenomenon because this is possible due to formation of a small electrochemical cell over rough surface of iron and the following redox reaction takes place there in that cell-
Oxidation Fe(s) → Fe2+(aq) + 2e-
Reduction O2+ 4H+ 4e– → 2H2O
e- + ½ O2 + 2H2O +2Fe2+→Fe2O3 + 4H+
Question. We expand crore of Rupees and even thousands of lives every year due to corrosion. How can be preventing it. Explain.
Answer: (i) By Galvanization: Coating of a less reactive metal with a more reactive metal e.g.- coating of iron surface with Zn to prevent rusting of iron.
(ii) By greasing /oiling (to keep away the object from the contact of air & moisture.)
(iii)By painting (to keep away the object from the contact of air & moisture
| CBSE Class 11 Chemistry Basic Concepts Of Chemistry Notes Set A |
| CBSE Class 11 Chemistry Basic Concepts Of Chemistry Notes Set B |
Important Practice Resources for Class 11 Chemistry
CBSE Class 11 Chemistry Chapter 7 Redox Reactions Notes
Students can use these Revision Notes for Chapter 7 Redox Reactions to quickly understand all the main concepts. This study material has been prepared as per the latest CBSE syllabus for Class 11. Our teachers always suggest that Class 11 students read these notes regularly as they are focused on the most important topics that usually appear in school tests and final exams.
NCERT Based Chapter 7 Redox Reactions Summary
Our expert team has used the official NCERT book for Class 11 Chemistry to design these notes. These are the notes that definitely you for your current academic year. After reading the chapter summary, you should also refer to our NCERT solutions for Class 11. Always compare your understanding with our teacher prepared answers as they will help you build a very strong base in Chemistry.
Chapter 7 Redox Reactions Complete Revision and Practice
To prepare very well for y our exams, students should also solve the MCQ questions and practice worksheets provided on this page. These extra solved questions will help you to check if you have understood all the concepts of Chapter 7 Redox Reactions. All study material on studiestoday.com is free and updated according to the latest Chemistry exam patterns. Using these revision notes daily will help you feel more confident and get better marks in your exams.
You can download the teacher prepared revision notes for CBSE Class 11 Chemistry Redox Reactions Notes Set E from StudiesToday.com. These notes are designed as per 2025-26 academic session to help Class 11 students get the best study material for Chemistry.
Yes, our CBSE Class 11 Chemistry Redox Reactions Notes Set E include 50% competency-based questions with focus on core logic, keyword definitions, and the practical application of Chemistry principles which is important for getting more marks in 2026 CBSE exams.
Yes, our CBSE Class 11 Chemistry Redox Reactions Notes Set E provide a detailed, topic wise breakdown of the chapter. Fundamental definitions, complex numerical formulas and all topics of CBSE syllabus in Class 11 is covered.
These notes for Chemistry are organized into bullet points and easy-to-read charts. By using CBSE Class 11 Chemistry Redox Reactions Notes Set E, Class 11 students fast revise formulas, key definitions before the exams.
No, all study resources on StudiesToday, including CBSE Class 11 Chemistry Redox Reactions Notes Set E, are available for immediate free download. Class 11 Chemistry study material is available in PDF and can be downloaded on mobile.

