Download the latest CBSE Class 11 Chemistry Organic Chemistry Notes Set D in PDF format. These Class 11 Chemistry revision notes are carefully designed by expert teachers to align with the 2025-26 syllabus. These notes are great daily learning and last minute exam preparation and they simplify complex topics and highlight important definitions for Class 11 students.
Chapter-wise Revision Notes for Class 11 Chemistry Chapter 8 Organic Chemistry Some Basic Principles & Techniques
To secure a higher rank, students should use these Class 11 Chemistry Chapter 8 Organic Chemistry Some Basic Principles & Techniques notes for quick learning of important concepts. These exam-oriented summaries focus on difficult topics and high-weightage sections helpful in school tests and final examinations.
Chapter 8 Organic Chemistry Some Basic Principles & Techniques Revision Notes for Class 11 Chemistry
Organic compounds are the hydrocarbons and their derivatives and organic chemistry is that branch of chemistry that deals with the study of these compounds
Tetravalency of carbon
The atomic number of Carbon is 6 and its electronic configuration is 2,4 i.e. it has 4 valence electrons. Thus carbon is always tetra covalent, i.e. it forms 4 covalent bonds with other atoms
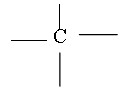
Due to tetravalency of carbon it has a tetrahedron shape.
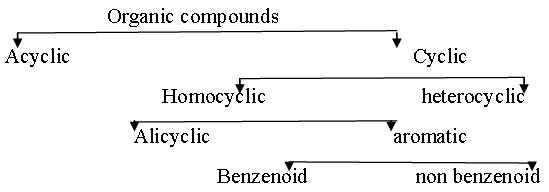
Catenation- The self linking property of carbon is known as catenation. This is the main reason of existence of such large number of compounds Classification of organic compounds
Functional groups: A functional group may be defined as an atom or a group of atoms present in a molecule which largely determines the chemical properties.
| CLASS OF ORGANIC | NAME OF FUNCTIONAL | STRUCTURE | ||
| COMPOUNDS | GROUP | |||
| Alkenes | double bond | =C=C= | ||
| Alkynes | triple bond | – C Ξ C – | ||
| Halogens | halogen | – X ( F, Cl ,Br, I ) | ||
| Alcohols | hydroxyl | -OH | ||
| Aldehydes | aldehydic (formal) | -CHO | ||
| Carboxylic acids | carboxyl | -COOH | ||
| Acid amides | amides | – CONH2 | ||
| amino | 0 |
Homologous Series
Homologous series is defined as a family or group of structurally similar organic compounds all members of which contain the same functional group, show a gradation in physical and similarity in chemical properties and any two adjacent members of which differ by -CH2 group. The individual members of this group are called homologues and the phenomenon is called homology.
Nomenclature Of Organic Compounds
Organic chemistry deals with millions of compounds. In order to clearly identify them, a systematic method of naming known as IUPAC system of nomenclature is adopted. The names are such that the listener can deduce the structure from it. The IUPAC name consists of three parts:
Prefix Word root Suffix
EX: 3 methlyoctane
Nomenclature Of Alkanes
Straight chain alkanes:
The names of such compounds is based on their chain structure, and end with suffix‘-ane’ and carry a prefix indicating the number of carbon atoms present in the chain.
Branched chain hydrocarbons:
1.) The longest carbon chain in the molecule is identified.
2.) The numbering is done in such a way that the branched carbon atoms get the lowest possible value.
3.) The names of the alkyl groups attached as a branch are then prefixed to the name of the parent alkane and its position is indicated by numbers.
4.) The lower number is given to the first in alphabetical order.
5.) The carbon atom of the branch that attaches to the root alkane is numbered 1.
Organic compounds having Functional Groups:
The longest chain of carbon atoms containing the functional groups is numbered in such a way that the functional group attached to the carbon atom gets the lowest possible number in the chain.
When there are more functional groups then a priority order is followed as:
-COOH, -SO3H, -COOR, COCl, -CONH2, -CN, -HC=O, =C=O, -OH, -NH2, =C=C=,-CΞ C-.
Isomerism
Two or more compounds having the same molecular formula but different physical and chemical properties are called isomers and this phenomenon is called isomerism.
Chain isomerism: When two or more compounds having same molecular formula but different carbon skeletons are referred to as chain isomers.
Position Isomerism : Compounds which have the same structure of carbon chain but differ in position of double or triple bonds or functional group are called position isomers and this phenomenon is called Position Isomerism. e g
CH3 – CH2 – CH=CH2 CH3 – CH = CH – CH3
Functional Isomerism : Compounds which have the same molecular formula but different functional group are called functional isomers and this phenomenon is called functional Isomerism. e g
CH3 – CH2 – OH CH3 – O – CH3
Metamerism : It is due to the presence of different alkyl groups on either side of functional group in the molecule. Ex. C4H10O represents C2H5OC2H5 and CH3OC3H7.
Fission Of Covalent Bond
Heterolytic cleavage : In this cleavage the bond breaks in such a way that the shared pair of electron remains with one of the fragments.
H3C → Br +CH3 + Br-
Homolytic Cleavage: In this cleavage the shared pair of electron goes with each of the bonded atom.
R – X → R. + X.
Alkyl free radical
Nucleophiles : A reagent that brings an electron pair is called nucleophile ie nucleus seeking e g -OH , -CN
Electrophiles: A reagent that takes away electron pair is called electrophile I e electron seeking e g > C= O , R3C – X
Inductive Effect: The displacement of the electron along the chain of the carbon atoms due to presence of an atom or group at the end of the chain.
ɗ+++ ɗ ++ ɗ+
CH3– C H2 CH2 Cl
Resonance Effect : The polarity produced in the molecule by the interaction of two pi bonds or between a pi bond and lone pair of electron present on an adjacent atom.
There are two types of resonance effect:
1) Positive resonance effect : In this effect the transfer of electrons is away from an atom or substituent group attached to the conjugated system.
The atoms or groups which shows +R effect are halogens,-OH , -OR,- NH2
2) Negative resonance effect : In this effect the transfer of electrons is towards the atom or substituent group attached to the conjugated system.
The atoms or groups which shows -R effect are –COOH , -CHO , -CN
Methods Of Purification Of Organic Compounds :
Sublimation : This method is used to separate the sublimable compounds from non sublimable compounds.
Crystallisation : This method is based on the difference in the solubilities of compound and impurities in a suitable solvent. The impure compound is dissolved in solvent at heated at higher temp .on cooling the hot and conc solution pure compounds crystallizes out.
Distillation: This method is used to separate volatile liquids from non volatile liquids and liquids having sufficient difference in their boiling points.
Fractional distillation : If the boiling points of two liquids is not much , they are separated by this method.
Distillation under reduced pressure : This method is used to purify liquids having high boiling points and decomposes at or below their boiling points.
Steam distillation : This method is used to separate substances which are steam volatile and are immiscible with water.
Differential Extraction: When an organic compound is present in an aqueous medium it is separated by shaking it with organic solvent in which it is more soluble than in water. The aqueous solution is mixed with organic solvent in a separating funnel and shaken for sometimes and then allowed to stand for some time. when organic solvent and water form two separate layers the lower layer is run out by opening the tap of funnel and organic layer is separated. the process is repeated several times and pure organic compound is separated.
Chromatography : This technique is used to separate mixtures in to their components ,purify the compounds and test the purity of compounds. It is classified as
Adsorption Chromatography : It is based on the fact that different compounds are adsorbed on an adsorbent to different degrees. Silica jel or alumina is used as adsorbents.
Partition Chromatography : It is based on the continuous differential portioning of components of a mixture between stationary and mobile phase
Qualitative Analysis Of Organic Compounds
Detection of Carbon and Hydrogen : The Carbon and Hydrogen present in the Organic compound is detected by heating the compound with Copper II oxide in a hard glass tube when carbon present in the compound is oxidized to CO2 which can be tested with lime Water and Hydrogenis converted to water which can be tested with anhydrous copper sulphate which turns blue.
C + CuO → 2Cu + CO2
2 H +CuO → Cu + H2O
CO2 +Ca (OH )2 → CaCO3 + H2O
5H2O + CuSO4 → CuSO4. 5H2O
Detection Of Other Elements
Sodium Fusion Extract: A small piece of dry Sodium metal is heated with a organic compound in a fusion tube for 2 -3 minutes and the red hot tube is plunged in to distilled water contained in a china dish. The contained of the china dish is boiled ,cooled and filtered. The filtrate is known as Sodium fusion extract.
Test for Nitrogen : The sodium fusion extract is boiled with iron II sulphate and then acidified with Concsulphuric acid , the formation of Prussian blue colour confirms the presence of nitrogen.
Test for Sulphur: the sodium fusion extract is acidified with acetic acid and lead acetate is added to it. A black precipitate of lead sulphide indicates the presence of sulphur.
S2- + Pb2+ → PbS
Black
Test for halogens: The sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. A white precipitate, soluble in ammonium hydroxide shows the presence of chlorine, a yellowish ppt. sparingly soluble in ammonium hydroxide shows the presence of bromine, a yellowish ppt. insoluble in ammonium hydroxide shows the presence of iodine.
X– + Ag+ → AgX
Quantitive Analysis (Carbon and Hydrogen)
Let the mass of organic compound be m g. Mass of water and carbon dioxide produced be m1 and m2 g respectively;
% of carbon = 12 x m2 x 100 / 44 x m
% of hydrogen = 2 x m1 x 100 / 18 x m
Nitrogen
Dumas Method: A known mass of organic compound is heated with excess of CuO in an atmosphere of CO2, when nitrogen of the organic compound is converted into N2 gas. The volume of N2 thus obtained is converted into STP and the percentage of nitrogen determined by applying the equation:
Volume of Nitrogen at STP = P1V1 x 273 / 760 x T1
%N = 28 x vol of N2 at STP x 100 / 22400 x mass of the substance taken
Kjeldahl’s Method: A known mass of organic compound is heated with conc. H2SO4 in presence of K2SO4 and little CuSO4 or Hg in a long necked flask called Kjeldahl’s flask when nitrogen present in the organic compound is quantitatively converted into (NH4)2SO4. (NH4 )2SO4 thus obtained is boiled with excess of NaOH solution to liberate NH3 gas which is absorbed in a known excess of a standard acid such as H2SO4 or HCl.
The vol of acid unused is found by titration against a standard alkali solution. From the vol of the acid used, the percentage of nitrogen is determined by applying the equation,
%N= 1.4 x Molarity of the acid x Basicity of the acid x Vol of the acid used / Mass of the substance taken
Halogens
Carius method:
A known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass test tube known as carius tube in a furnace. Carbon and hydrogen present in the compound are oxidized to carbon dioxide and water. The halogen present forms the corresponding silver halide. It is filtered, dried, and weighed.
Let the mass of the organic compound taken = m g
Mass of AgX formed = m1 g
1 mol of AgX contains 1 mol of of X
Mass of halogen in m1 g of AgX
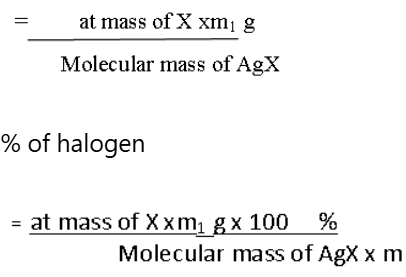
Sulphur
Let the mass of the organic compound taken = m g
Mass of BaSO4 formed = m1 g
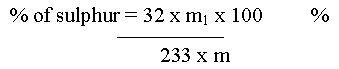
Phosphorous
Let the mass of the organic compound taken = m g
Mass of ammonium phosphomoly date = m1 g
![]()
Oxygen
Let the mass of the organic compound taken = m g
Mass of CO2 = m1 g
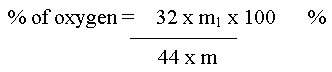
Question. Suggest a method to purify a liquid which decomposes at its boiling point.
Answer: The process Distillation Under reduced pressure is used to purify a liquid which decomposes at its boiling point.
Question. How will you separate a mixture of O-nitrophenol and p- nitrophenol ?
Answer: O-nitrophenol is steam volatile therefore it can be separated by Steam distillation.
Question. Lassaigne’s test is not shown by diazonium Salt. Why?
Answer: On heating diazonium Salts loses Nitrogen and could not fuse with the Sodium metal therefore diazonium Salt do not show Positive Lassaigne’s test for nitrogen.
Question. Alcohols are weaker acids than Water, Why ?
Answer: The alkyl group in alcohols has + I effect due to which electron density is increases on Oxygen atom which makes the release of hydrogen ion more difficult from alcohol .R → O → H
Question. Why is nitric acid is added to Sodium extract before adding Silver nitrate for testing halogens ?
Answer: Nitric acid is added to decompose NaCN and Na2S
NaCN + HNO3 → NaNO3 + HCN
Na2S + 2HNO3 → 2NaNO3 + H2S
Question. which of the two O2NCH2CH2 – or CH3CH2O – is expected to be more stable and why ?
Answer: NO2 group has –I effect and disperse the negative charge on Oxygen atom
O2N ←CH2← CH2O–
Question. Arrange the following in increasing Order of Stability ;
(CH3)3C + , CH3CH2CH2C+H2 , CH3CH2C+HCH3 ,CH3C+H2 , CH3CH2C+H2
Answer: CH3C+H2 < CH3CH2C+H2< CH3CH2CH2C+H2<CH3CH2C+HCH3< (CH3 )3C +
Question. Write the IUPAC name of the following
CH3 CH CH CH2 CH3
Answer: 2,3Dimethylpentane
Question. Write the hybridized state of C atoms in the following
CH2 = CH – C Ξ N
Answer: sp2sp2sp
CH2 = CH – C Ξ N
Question. Give the IUPAC name of the following compound.
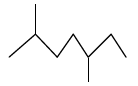
Answer: 2,5Dimethylheptane
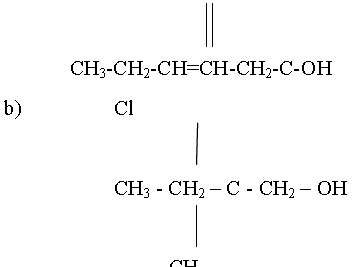
Question. Draw the Structures of the following compounds.
A) Hex-3-enoic acid
b) 2-chloro-2-methylbutan-1-ol
Answer: a)
Question. Explain Inductive effect with example.
Answer: Inductive Effect: The displacement of the electron along the chain of the carbon atoms due to presence of an atom or group at the end of the chain.
δ+++ δ++ δ+
CH3→ C H2 → CH2 →Cl
Question. Explain why (CH3)3C+ is more stable than CH3C+H2.
Answer: (CH3)3C+ has nine alpha hydrogens and has nine hyperconjugation structures while CH3C+H2 has three alpha hydrogens and has three hyperconjugation structures, therefore (CH3)3C+ is more stable than CH3C+H2
Question. Give the number of Sigma and pi bonds in the following molecules
a) CH3NO2
b) HCONHCH3
Answer: a) 6 Sigma and 1 pi bond
b) 8 Sigma and 1 pi bond
Question. Write the condensed and bond line formula of 2,2,4-Trimethylpentane
Answer: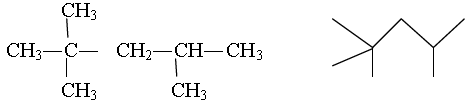
Question. How Sodium fusion extract is prepared ?
Answer: A small piece of dry Sodium metal is heated with a organic compound in a fusion tube for 2 -3 minutes and the red hot tube is plunged in to distilled water contained in a china dish. The contained of the china dish is boiled ,cooled and filtered. The filtrate is known as Sodium fusion extract.
Question. Explain the principle of paper chromatography.
Answer: Paper chromatography is based on the difference in the rates at which the components of a mixture are adsorbed. The material on which different components are adsorbed is called Stationary phase which is generally made up of alumina, silicate or activated charcoal. The mixture to be separated is dissolved in a suitable medium and it is called moving phase. The moving phase is run on the Stationary phase , the different compounds are adsorbed on stationary phase at different rates.
Question. Why is an organic compound fused with Sodium for testing nitrogen, halogens and sulphur ?
Answer: On fusing with sodium metal the elements presents in an organic compounds are converted in to sodium salts which are water soluble which can be filtered and detected by the respective tests.
Question. It is not advisable to use sulphuric acid in place of acetic acid for acidification while testing sulphur by lead acetate test. Give reason
Answer: Lead acetate on reacting with sulphuric acid will give a white ppt of lead sulphatewhih interfere in the detection of sulphur.
(CH3COO)2Pb + H2SO4 → PbSO4 + 2CH3COOH
Question. Under what conditions can the process of steam distillation is used ?
Answer: Steam distillation is used to purify the liquids which are steam volatile and water and the liquid are not miscible with each other.
Question. In an estimation of sulphur by carius method 0.468 g of an organic compound gave 0.668 g of barium sulphate. Find the percentage of sulphur in the compound.
Answer: Mass of the compound = 0.468 g
Mass of the barium sulphate = 0.668 g
% of sulphur = 32 X Mass of barium sulphate X 100 / 233 X Mass of the compound
= 32 x 0.668×100 / 233 x0.468
= 19.60 %
Question. Which bond is more polar in the following pairs of molecules.
a) H3C-H, H3C-Br
b) H3C-NH2, H2C-OH
c) H3C-OH, H3C-SH
Answer: a) C-Br because Br is more electronegative than H
b) C-O because O is more electronegative than N
c) C-O because O is more electronegative than S
Question. Define Isomerism. Explain position Isomerism and Functional Isomerism with examples.
Answer: Two or more compounds having the same molecular formula but different physical and chemical properties are called isomers and this phenomenon is called isomerism.
Position Isomerism : Compounds which have the same structure of carbon chain but differ in position of double or triple bonds or functional group are called position isomers and this phenomenon is called Position Isomerism. e g
CH3 – CH2 – CH=CH2 CH3 – CH = CH – CH3
Functional Isomerism :Compounds which have the same molecular formula but different functional group are called functional isomers and this phenomenon is called functional Isomerism. e g
CH3 – CH2 – OH CH3 – O – CH3
Question. write the IUPAC names of the following compounds.
O O
ll ll
A. CH3 – CH2 –C – CH2 – C – CH3
B HC Ξ C – CH = CH – CH – CH2
C Cl CH2CH2CH2CH2Br
Answer: A hexane 2,4dione
B hexa-1,3 – dien-5 – yne
C 1 – bromo – 4 – chlorobutane
Question. Define Homologous series. Write the general formula of alkane, alkene and alkynes.
Answer: Homologous Series : It is defined as group of similar organic compounds which contains the similar functional groups and the two adjacent members of the series is differ by a –CH2 group.
Alkanes CnH2n+2
Alkenes CnH2n
Alkynes CnH2n-2
Question. How many Sigma and pi bonds are present in the following molecules .
A HC Ξ CCH = CHCH3
B CH2 = C = CHCH3
Answer: A Sigma bonds = 10 pi bonds = 3
B Sigma bonds = 9 pi bonds = 2
Question. Define functional groups. Write the general formula of Carboxylic acids acid chlorides.
Answer: Functional Groups :It is an atom or group of atoms bonded together in a unique manner which is usually the site of chemical reactivity in an organic molecule. e g CH3OH
General formula of Carboxylic acids : CnH2n+1COOH
General formula of acid chlorides : RCOCl
Question. Write a shirt note on differential extraction.
Answer: When an organic compound is present in an aqueous medium it is separated by shaking it with organic solvent in which it is more soluble than in water. The aqueous solution is mixed with organic solvent in a separating funnel and shaken for sometimes and then allowed to stand for some time .when organic solvent and water form two separate layers the lower layer is run out by opening the tap of funnel and organic layer is separated. the process is repeated several times and pure organic compound is separated.
Question. How carbon and Hydrogen is detected in a organic compounds.
Answer: The Carbon and Hydrogen present in the Organic compound is detected by heating the compound with Copper II oxide in a hard glass tube when carbon present
in the compound is oxidized to CO2 which can be tested with lime Water and Hydrogenis converted to water which can be tested with anhydrous copper sulphate which turns blue.
C + CuO → 2Cu + CO2
2 H +CuO → Cu + H2O
CO2 +Ca (OH) → 2CaCO3 + H2O
5H2O + CuSO4 → CuSO4.5H2O
Question. Write a short note on Resonance effect.
Answer: Resonance Effect : The polarity produced in the molecule by the interaction of two pi bonds or between a pi bond and lone pair of electron present on an adjacent atom.
There are two types of resonance effect:
1. Positive resonance effect: In this effect the transfer of electrons is away from an atom or substituent group attached to the conjugated system.
The atoms or groups which shows +R effect are halogens,- OH , -OR,- NH2
2. Negative resonance effect: In this effect the transfer of electrons is towards the atom or substituent group attached to the conjugated system.
The atoms or groups which shows -R effect are – COOH , -CHO , -CN
Question. Differentiate between the principle of estimation of nitrogen in an organic
compound by i) Dumas method ii) Kjeldahl’s method.
Answer: DUMAS METHOD: A known mass of organic compound is heated with excess of CuO in an atmosphere of CO2, when nitrogen of the organic compound is converted into N2 gas. The volume of N2 thus obtained is converted into STP and the percentage of nitrogen determined by applying the equation:
Volume of Nitrogen at STP = P1V1 x 273 / 760 x T1
%N = 28 x vol of N2 at STP x 100 / 22400 x mass of the substance taken
Kjeldahl’s Method: A known mass of organic compound is heated with conc. H2SO4 in presence of K2SO4 and little CuSO4 or Hg in a long necked flask called Kjeldahl’s flask when nitrogen present in the organic compound is quantitatively converted into (NH4)2SO4. (NH4)2SO4 thus obtained is boiled with excess of NaOH solution to liberate NH3 gas which is absorbed in a known excess of a standard acid such as H2SO4 or HCl.
The vol of acid unused is found by titration against a standard alkali solution. From the vol of the acid used, the percentage of nitrogen is determined by applying the equation,
%N= 1.4 x Molarity of the acid x Basicity of the acid x Vol of the acid used / Mass of the substance taken
Question. A sample of 0.50g of organic compound was treated according to Kjeldahl’s method. The ammonia evolved was absorbed in 50mL of 0.5M H2SO4 . The residual acid required 60mL of 0.5M solution of NaOH for neutralization. Find the percentage composition of nitrogen in the compound.
Answer: the vol of H2SO4 used.
Vol of acid taken=50mL of 0.5M H2SO4 = 25mL of 1M H2SO4
Vol of alkali used for neutralization of excess acid= 60 mL of 0.5m NaOH = 30mL of 1M NaOH
Now 1 mole of H2SO4 neutralizes 2 moles of NaOH
(i.e. H2SO4 + 2 NaOH → Na2SO4 + 2H2O)
… 30 mL of 1M NaOH = 15mL of 1M H2SO4
% of nitrogen.
1 mole of H2SO4 neutralizes 2 moles of NH3 … 10mL of 1M H2SO4 = 20mL of 1M NH3
But 1000 mL of 1M NH3 contain N=14g.
20 ml of 1M NH3 will contain nitrogen = 14 x 20 / 1000
But this amount of nitrogen is present in 0.5 g of organic compound..
. % of N = 14 x 20 x 100 / 1000 x 0.5 = 56.0
Question. You have a mixture of three liquids A, B , C. there is a large difference in the boiling point of A and the rest two liquids. Boiling points of liquids B and C are quite close. Liquid A boils at higher temperature than B and C and boiling point of B is lower than C. How will you separate the components of the mixture.
Answer: Since the boiling point of liquid A is much higher than those of liquids B and C , therefore separate liquid A by simple distillation. Since boiling points of liquids B and C are quite close but much lower than liquid A therefore mixture of B and C will distil together leaving behind A. on further heating A will distil over. Now place the mixture of liquids B and C in a flask fitted with fractionating column. Since the b.p. of liquid B is lower than that of C , on fractional distillation first liquid B will distil over and than liquid C.
Question. Explain hyperconjugation effect. How does hyperconjugation effect explain the stability of alkenes?
Answer: The relative stability of various classes of carbonium ions may be explained by the number of no – bond resonance structures that can be written for them. Such structures are obtained by shifting the bonding electrons from an adjacent C-H bond to the electron deficient carbon so the positive charge originally on carbon is dispersed to the hydrogen. This manner of electron release by assuming no bond character in the adjacent C-H bond is called Hyperconjugation. Greater the hyperconjugation greater will be the stability of alkenes.
![]()
Question. In DNA and RNA nitrogen is present in the ring system. Can kjeldahl method be used for the estimation of nitrogen present in these ?give reasons
Answer: In DNA and RNA nitrogen is present in hetrocyclicrings. Kjeldahl method can not be used to estimate nitrogen present in the ring because cannot be completely converted in to (NH4)2SO4 during digestion. Therefore Kjeldahl method can not be used to estimate nitrogen present in DNA and RNA
Question. 1.216 g of an organic compound was Kjeldahlised and the ammonia evolved was absorbed in 100 mL 1N H2SO4 . The remaining acid solution was made upto500ml by addition of water. 20ml of this dilute solution required 32mL of N/10 caustic soda solution for complete neutralization. Calculate the percentage of nitrogen in the organic compound.
Answer: 20 ml of dil. Unreacted H2SO4 = 32mL of N/10 NaOH sol
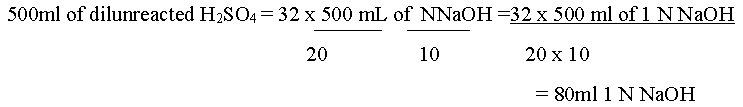
But 80ml 1 N NaOH= 80ml 1 N NaOH So, acid left unused = 80ml 1 N H2SO4 4 Acid used =(100 – 80) = 20ml 1 N H2SO4
%N= 1.4 x Normality of the acid x Vol of the acid used / Mass of the substance taken
= 1.4 x 1 x 20 / 1.216 = 23.026
| CBSE Class 11 Chemistry Basic Concepts Of Chemistry Notes Set A |
| CBSE Class 11 Chemistry Basic Concepts Of Chemistry Notes Set B |
Important Practice Resources for Class 11 Chemistry
CBSE Class 11 Chemistry Chapter 8 Organic Chemistry Some Basic Principles & Techniques Notes
Students can use these Revision Notes for Chapter 8 Organic Chemistry Some Basic Principles & Techniques to quickly understand all the main concepts. This study material has been prepared as per the latest CBSE syllabus for Class 11. Our teachers always suggest that Class 11 students read these notes regularly as they are focused on the most important topics that usually appear in school tests and final exams.
NCERT Based Chapter 8 Organic Chemistry Some Basic Principles & Techniques Summary
Our expert team has used the official NCERT book for Class 11 Chemistry to design these notes. These are the notes that definitely you for your current academic year. After reading the chapter summary, you should also refer to our NCERT solutions for Class 11. Always compare your understanding with our teacher prepared answers as they will help you build a very strong base in Chemistry.
Chapter 8 Organic Chemistry Some Basic Principles & Techniques Complete Revision and Practice
To prepare very well for y our exams, students should also solve the MCQ questions and practice worksheets provided on this page. These extra solved questions will help you to check if you have understood all the concepts of Chapter 8 Organic Chemistry Some Basic Principles & Techniques. All study material on studiestoday.com is free and updated according to the latest Chemistry exam patterns. Using these revision notes daily will help you feel more confident and get better marks in your exams.
You can download the teacher prepared revision notes for CBSE Class 11 Chemistry Organic Chemistry Notes Set D from StudiesToday.com. These notes are designed as per 2025-26 academic session to help Class 11 students get the best study material for Chemistry.
Yes, our CBSE Class 11 Chemistry Organic Chemistry Notes Set D include 50% competency-based questions with focus on core logic, keyword definitions, and the practical application of Chemistry principles which is important for getting more marks in 2026 CBSE exams.
Yes, our CBSE Class 11 Chemistry Organic Chemistry Notes Set D provide a detailed, topic wise breakdown of the chapter. Fundamental definitions, complex numerical formulas and all topics of CBSE syllabus in Class 11 is covered.
These notes for Chemistry are organized into bullet points and easy-to-read charts. By using CBSE Class 11 Chemistry Organic Chemistry Notes Set D, Class 11 students fast revise formulas, key definitions before the exams.
No, all study resources on StudiesToday, including CBSE Class 11 Chemistry Organic Chemistry Notes Set D, are available for immediate free download. Class 11 Chemistry study material is available in PDF and can be downloaded on mobile.

