Read and download the CBSE Class 10 Chemistry Periodic Classification Of Elements Worksheet Set E in PDF format. We have provided exhaustive and printable Class 10 Science worksheets for Chapter 5 Periodic Classification of Elements, designed by expert teachers. These resources align with the 2025-26 syllabus and examination patterns issued by NCERT, CBSE, and KVS, helping students master all important chapter topics.
Chapter-wise Worksheet for Class 10 Science Chapter 5 Periodic Classification of Elements
Students of Class 10 should use this Science practice paper to check their understanding of Chapter 5 Periodic Classification of Elements as it includes essential problems and detailed solutions. Regular self-testing with these will help you achieve higher marks in your school tests and final examinations.
Class 10 Science Chapter 5 Periodic Classification of Elements Worksheet with Answers
Question: According to Mendeleev’s Periodic Law, the elements were arranged in the periodic table in the order of
a) increasing atomic number
b) decreasing atomic number
c) increasing atomic masses
d) decreasing atomic masses
Answer: c
Question: Which of the following elements has 2 shells and both are completely filled?
a) Helium
b) Neon
c) Calcium
d) Boron
Answer: b
Question: The properties of Eka-aluminium predicted by Mendeleev are the same as the properties of later discovered element:
a) Scandium
b) Germanium
c) Gallium
d) Aluminium
Answer: c
Question: The arrangement of elements in the Modern Periodic Table is based on their
a) increasing atomic mass in the period
b) increasing atomic number in the horizontal rows
c) increasing atomic number in the vertical columns
d) increasing atomic mass in the group
Answer: b
Question: Element ‘X’ forms a chloride with the formula XCl₂, which is a solid with high melting point. X would most likely be in the same group as:
a) Si
b) Mg
c) Al
d) Na
Answer: b
Question: A metal ‘M’ is in the first group of the Periodic Table. What will be the formula of its oxide?
a) MO
b) M₂O
c) M₂O₃
d) MO₂
Answer: b
Question: An element X has mass number 40 and contains 21 neutrons. To which group does it belong?
a) Group 1
b) Group 4
c) Group 2
d) Group 3
Answer: a
Question: What is the other name for group 18 elements?
a) Noble gases
b) Alkali metals
c) Alkali earth metals
d) Halogens
Answer: a
Question: The atom with electronic configuration 2, 8, 7 would be chemically similar to
a) ⁷N
b) ¹⁵P
c) ¹¹Na
d) ⁹F
Answer: d
Question: Which of the following are the characteristics of isotopes of an element?
(i) Isotopes of an element have same atomic masses
(ii) Isotopes of an element have same atomic number
(iii) Isotopes of an element show same physical properties
(iv) Isotopes of an element show same chemical properties
(a) (i), (iii) and (iv)
(b) (ii), (iii) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer: d
Assertion Reason type Questions
In the following questions a statement of assertion followed by a statement of reason is given.
Choose the correct answer out of the following choices.
(A) Both assertion and reason are true and the reason is the correct explanation of assertion.
(B) Both assertion and reason are true and the reason is not the correct explanation of assertion.
(C) Assertion is true but the reason is false.
(D) Both assertion and reason are false.
(E) Assertion is false but reason is true.
Question: Assertion: Properties of an atom and its corresponding ion remains the same.
Reason: Electronic configurations of both atom and ion remain same.
Answer: d
Question: Assertion: Group 18 elements are inert.
Reason: They have completely filled valence shell.
Answer: a
Question: Assertion: According to Mendeleev, periodic properties of elements is a function of their atomic number.
Reason: Atomic number is equal to the sum of number of protons and neutrons.
Answer: d
Question: Assertion: Li, Na, K belong to Dobereiner’s triads.
Reason: The atomic mass of Na is the average of atomic masses of Li and K.
Answer: a
Question: Assertion: Mendeleev left some gaps in his Periodic Table.
Reason: Mendeleev believed that some elements would be discovered later.
Answer: a
Case based Questions
Today, 118 elements are known, the first 94 of which occur in nature. Of the 94 natural elements, eighty are stable. The periodic table is a graphic description of the periodic law, which states that the properties and atomic structures of the chemical elements are a periodic function of their atomic number.
Elements are placed in the periodic table by their electron configurations, which exhibit periodic recurrences that explain the trends of properties across the periodic table. As we go across a period from left to right, we add a proton to the nucleus and an electron to the valence shell with each successive element. As we go down the elements in a group, the number of electrons in the valence shell remains constant, but the principal quantum number increases by one each time.
An understanding of the electronic structure of the elements allows us to examine some of the properties that govern their chemical behavior. These properties vary periodically as the electronic structure of the elements changes.
They are
(1) size (radius) of atoms and ions,
(2) ionization energies, and
(3) electron affinities.
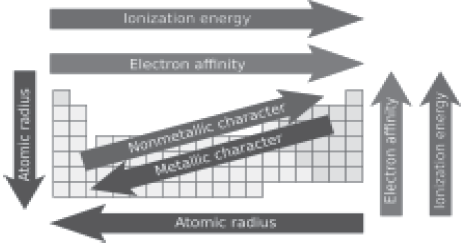
Question: Which of the following set of elements is written in order of their increasing metallic character?
a) Na, Li, K
b) C, O, N
c) Mg, Al, Si
d) Be, Mg, Ca
Answer: d
Question: Which of the following is the correct order of the atomic radii of the elements oxygen, fluorine and nitrogen?
a) O < F < N
b) N < F < O
c) O < N < F
d) F < O < N
Answer: d
Question: What happens to tendency to gain electron in a period?
a) Increases
b) Decreases
c) Remaining same
d) First increases then decreases
Answer: a
Question: Which of the following elements would lose an electron easily?
a) Mg
b) Na
c) K
d) Ca
Answer: c
Question: Atomic size decreases from left to right in a period because
a) Effective nuclear charge increases
b) Number of shells remains the same
c) Force of attraction between the nucleus and valence electrons increases
d) All of these
Answer: d
Descriptive Questions Of 1 Mark
Question: Write the formulae of chlorides of Eka-silicon and Eka-aluminium, the elements predicted by Mendeleev.
Answer. Formula of Chloride of Eka Silicon is GeCl4 and that of Eka Aluminium is GaCl3
Question: State one reason for placing Mg and Ca in the same group of the periodic table.
Answer. They have same number of valence electrons and similar chemical properties.
Question: How does atomic size vary from left to right in a period?
Answer. Atomic size decreases from left to right in a period.
Question: State the modern periodic law of classification of elements.
Answer. It states that “the properties of the elements are periodic functions of their atomic numbers.”
Question: Name any three metalloids.
Answer. Boron, Silicon and Germanium.
Short Answer Type Questions
Question: How does the metallic character of elements change along a period of the periodic table from the left to the right and in a group from top to bottom why?
Answer. The metallic character goes on decreasing along a period from left to right because atomic size goes on decreasing therefore, tendency to lose electrons decreases. But in a group from top to bottom metallic character goes on increasing due to increase in size from top to bottom giving a tendency to lose electrons which are loosely held.
Question: (a) Electropositive nature of the element(s) increases down the group and decreases across the period
(b) Electronegativity of the element decreases down the group and increases across the period
(c) Atomic size increases down the group and decreases across a period (left to right)
(d) Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
(a) Name the most electropositive element among them
(b) Name the most electronegative element
(c) Name the element with smallest atomic size
(d) Name the element which is a metalloid
(e) Name the element which shows maximum valency
Answer. (a) Lithium (3)
(b) Fluorine (9)
(c) Fluorine (9)
(d) Boron (5)
(e) Carbon (6). Its valency is 4.
Question: How can the valency of an element be determined if its electronic configuration is known?
What will be the valency of an element of atomic number 9 (nine)?
Answer. It is the combining capacity of the element.
Generally, valency=number of valence electron or 8 – number of valence electron.
If the element has 1, 2, 3, 4 valence electrons, its valency will be 1, 2, 3, 4 respectively.
If the element has 5, 6, 7, 8 valence electrons, its valency will be 3, 2, 1, 0.
Element with atomic number 9 has electronic configuration 2, 7. So, its valency will be 1.
Question: The atomic numbers of three elements, X, Y and Z are 9,11 and 17 respectively. Which two of these elements will show similar chemical properties? Why?
Answer. Electronic configuration of X, Y and Z will be: X (9) : 2, 7 Y(11) : 2, 8, 1 Z(17) : 2, 8, 7 X and Z will show similar chemical properties due to same number of valence electrons.
Question: An element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of Ferrous sulphate crystals and are the major air pollutants.
(a) Identify the element X
(b) Write the electronic configuration of X
(c) Write the balanced chemical equation for the thermal decomposition of Ferrous sulphate crystals?
(d) What would be the nature (acidic/ basic) of oxides formed?
(e) Locate the position of the element in the Modern Periodic Table.
Answer.

Question: How does the valency of elements vary (a) in going down a group, and (b) in going from left to right in a period of the periodic table
Answer. (a) Valency remains the same in a group.
(b) Valency first goes on increasing from left to right in a period till middle of period, then Decreases
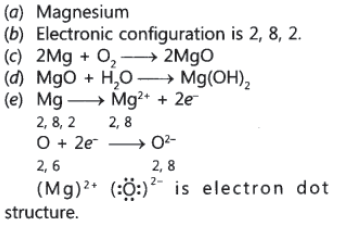
Question: An element is placed in 2nd Group and 3rd Period of the Periodic Table, burns in presence of oxygen to form a basic oxide.
(a) Identify the element
(b) Write the electronic configuration
(c) Write the balanced equation when it burns in the presence of air
(d) Write a balanced equation when this oxide is dissolved in water
(e) Draw the electron dot structure for the formation of this oxide
Answer.
Question: An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a
divalent halide.
(a) Where in the periodic table are elements X and Y placed?
(b) Classify X and Y as metal (s), non-metal (s) or metalloid (s)
(c) What will be the nature of oxide of element Y? Identify the nature of bonding in the compound formed
(d) Draw the electron dot structure of the divalent halide.
Answer.

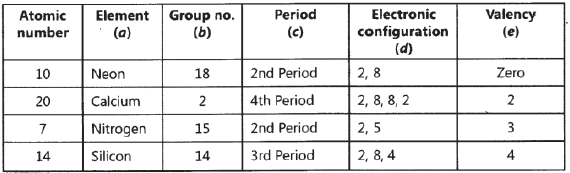
Question: Atomic number of a few elements are given below 10, 20, 7, 14
(a) Identify the elements
(b) Identify the Group number of these elements in the Periodic Table
(c) Identify the Periods of these elements in the Periodic Table
(d) What would be the electronic configuration for each of these elements?
(e) Determine the valency of these elements
Answer.
Question: (a) In the Mendeleev’s Periodic table, why does Argon with atomic mass 39.9 appear before Potassium having atomic mass 39.1? (b) Why is atomic number a more important property than atomic mass?
Answer. (a). It is because Mendeleev arranged elements giving more importance to the similarity in chemical properties of an element with the remaining members of the group .So Argon ,being an inert gas was placed along with inert gases and Potassium being an alkali metal is placed along with the other alkali metals. So Argon and Potassium are examples of anomalous pair.
(b) Atomic number gives us the electronic configuration which gives the number of valence electron which decides the chemical nature of the element.
Long Answer Type Questions
Question: Two elements X and Y belong to group 1 and 2 respectively in the same period of periodic table. Compare them with respect to:
(i) the number of valence electrons in their atoms;
(ii) their valencies;
(iii) metallic character;
(iv) the sizes of their atoms;
(v) the formulae of their oxides;
(vi) the formulae of their chlorides.
Answer. X and Y belong to same period, X belongs to group ‘1’. Y belongs to group ‘2’.
(i) Valence electron in X is 1 whereas valence electrons in Y are 2.
(ii) The valency of X is 1 whereas valency of Y is 2.
(iii) X is more metallic than Y because metallic character decreases on moving from left to right in a period.
(iv) The size of X is more than Y because size of the atom decreases on moving from left to right in a period.
(v) Oxide of X = X2O, Oxide of Y = YO
(vi) Chloride of X = XCl, Chloride of Y = YCl2
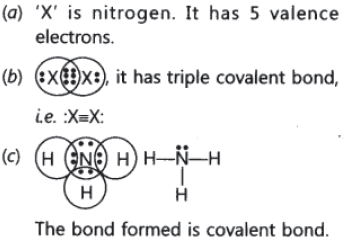
Question: An element X of group 15 exists as diatomic molecule and combines with hydrogen at 773 K in presence of the catalyst to form a compound, ammonia which has a characteristic pungent smell.
(a) Identify the element X. How many valence electrons does it have?
(b) Draw the electron dot structure of the diatomic molecule of X. What type of bond is formed in it?
(c) Draw the electron dot structure for ammonia and what type of bond is formed in it?
Answer.
Question: a. Calcium is an element with atomic number 20.
(i) Is it a metal or non-metal?
(ii) Will its size be more or smaller than that of potassium?
(iii) Write the formula of its chloride.
b. An element ‘X’ has mass number 35 and number of neutrons 18. Write atomic number and electronic configuration of ‘X’. Also write group number, period number and valency of ‘X’.
Answer. a .The electronic configuration of calcium (Z = 20) is 2, 8, 8, 2.
(i) Since it has only two valence electrons, it is present in group 2. It is a metal.
(ii) Both potassium (K) and calcium (Ca) are present in fourth period. Since atomic size decreases along a period, calcium is smaller in size.
(iii) The valency of calcium is 2. The formula of its chloride is CaCl2.
b. Atomic number of the element ‘X’ = 35 – 18 = 17
Gp17, period-3
Important Practice Resources for Class 10 Science
CBSE Science Class 10 Chapter 5 Periodic Classification of Elements Worksheet
Students can use the practice questions and answers provided above for Chapter 5 Periodic Classification of Elements to prepare for their upcoming school tests. This resource is designed by expert teachers as per the latest 2026 syllabus released by CBSE for Class 10. We suggest that Class 10 students solve these questions daily for a strong foundation in Science.
Chapter 5 Periodic Classification of Elements Solutions & NCERT Alignment
Our expert teachers have referred to the latest NCERT book for Class 10 Science to create these exercises. After solving the questions you should compare your answers with our detailed solutions as they have been designed by expert teachers. You will understand the correct way to write answers for the CBSE exams. You can also see above MCQ questions for Science to cover every important topic in the chapter.
Class 10 Exam Preparation Strategy
Regular practice of this Class 10 Science study material helps you to be familiar with the most regularly asked exam topics. If you find any topic in Chapter 5 Periodic Classification of Elements difficult then you can refer to our NCERT solutions for Class 10 Science. All revision sheets and printable assignments on studiestoday.com are free and updated to help students get better scores in their school examinations.
You can download the latest chapter-wise printable worksheets for Class 10 Science Chapter Chapter 5 Periodic Classification of Elements for free from StudiesToday.com. These have been made as per the latest CBSE curriculum for this academic year.
Yes, Class 10 Science worksheets for Chapter Chapter 5 Periodic Classification of Elements focus on activity-based learning and also competency-style questions. This helps students to apply theoretical knowledge to practical scenarios.
Yes, we have provided solved worksheets for Class 10 Science Chapter Chapter 5 Periodic Classification of Elements to help students verify their answers instantly.
Yes, our Class 10 Science test sheets are mobile-friendly PDFs and can be printed by teachers for classroom.
For Chapter Chapter 5 Periodic Classification of Elements, regular practice with our worksheets will improve question-handling speed and help students understand all technical terms and diagrams.

