These are the esters of fatty acids and glycerol. Again they are typed as:–
(a) Fats and Oils: (Natural lipids or true fats). These are triglycerides of fatty acid and glycerol.Fats which are liquid at room temperature are called oils. Oils with polyunsaturated fatty acids are called polyunsaturated e.g. sunflower oil, lower blood cholesterol.
(b) Fatty acids: Obtained by hydrolysis of fats. Formic acid is simplest fatty acid (HCOOH). These are of 2 types:–
(i) Saturated fatty acids: The fatty acids which do not have double bond in between carbon atoms.e.g. butyric acid, palmitic acid,hexanoic acid, etc. They have high melting points, solid at room temperature and increase blood cholesterol.
(ii) Unsaturated fatty acids: The fatty acids which have double bonds in carbon atoms. e.g. 8 hexadecanoic acid, 9 octadecanoic acid etc. They have lower melting points mostly found in plant fats, liquid at room temperature and lower the blood cholesterol.
(c) Waxes: These are simple lipids composed of one molecule of long chain fatty acid and long chain monohydric alcohol. Waxes have high melting point, insoluble in water, resistant to atmospheric oxidation, chemically inert and not digested by enzymes. They reduce rate of transpiration by making plant tissue water proof and work as excellent lubricant.
(a) Phospholipids: These contain phosphoric acid. It helps in transport, metabolism, blood clotting and permeability of cell membrane. It is a bipolar molecule i.e. phosphate containing end is hydrophilic whereas fatty acid molecules represent hydrophobic (non-polar tail).
(b) Glycolipids: These contain nitrogen and carbohydrate beside fatty acids. Generally found in white matter of nervous system. e.g. sesocine frenocin.
(c) Chromolipids : It includes pigmented lipids e.g. carotene.
(d) Aminolipids : Also known as sulpholipids. It contains sulphur and amino acids with fatty acid and glycerol. Cutin and suberin are also compound lipids resistant to water and also provide mechanical support in plants.
(i) Derived lipids: These are obtained by hydrolysis of simple and compound lipids.
(5) Functions of lipids
(a) Oxidation of lipids yields comparatively more energy in the cell than protein and carbohydrates. 1gm of lipids accounts for 39.1 KJ.
(b) The oil seeds such as groundnut, mustard, coconut store fats to provide nourishment to embryo during germination.
(c) They function as structural constituent i.e. all the membrane systems of the cell are made up of lipoproteins.
(d) Amphipathic lipids are emulsifier.
(e) It works as heat insulator.
(f) Used in synthesis of hormones.
(g) Fats provide solubility to vitamins A, D, E, and K.
Amino Acids
(1) Amino acids are normal components of cell proteins (called amino acid).
(2) They are 20 in number specified in genetic code and universal in viruses, prokaryotes and eukaryotes.
(3) Structure and Composition : Amino acids are basic units of protein and made up of C, H, O, N and sometimes S. Amino acids are organic acids with a carboxyl group (–COOH) and one amino group(-NH2) on the a -carbon atom. Carboxyl group attributes acidic properties and amino group gives basic ones. In solution, they serve as buffers and help to maintain pH. General formula is RCHNH2. COOH.
(4) Classification
Based on R-group of amino acids
(a) Simple amino acids: These have no functional group in the side chain. e.g. glycine, alanine ,leucine, valine etc.
(b) Hydroxy amino acids: They have alcohol group in side chain. e.g. threonine, serine, etc.
(c) Sulphur containing amino acids: They have sulphur atom in side chain. e.g. methionine,cystenine.
(d) Basic amino acids: They have basic group (-NH2) in side chain. e.g. lysine, arginine.
(e) Acidic amino acids: They have carboxyl group in side chain. e.g. aspartic acid, glutamic acid.
(f) Acid amide amino acids: These are the derivatives of acidic amino acids. In this group, one of the carboxyl group has been converted to amide (-CONH2). e.g. asparagine, glutamine.
(g) Heterocyclic amino acids: These are the amino acids in which the side chain includes a ring involving at least one atom other than carbon. e.g. tryptophan, histidine.
(h) Aromatic amino acids: They have aromatic group (benzene ring) in the side chain. e.g. phenylalanine, tyrosine, etc.
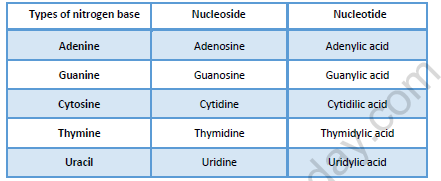
Nucleotides:
(1) Structurally a nucleotide can be regarded as a phosphoester of a nucleoside.
(2) A combination of nitrogens base and a sugar is called nucleoside and combination of a base, a sugar and phosphate group is known as nucleotide.
(3) Functions of nucleotides: Following are the major functions of nucleotides.
(a) Formation of nucleic acids: Different nucleotides polymerize together to form DNA and RNA.
(b) Formation of energy carrier: They help in formation of ATP,AMP, ADP, GDP, GTP, TDP,TTP, UDP, etc. which on breaking release energy.
(c) Formation of Coenzymes: Coenzymes like NAD, NADP, FMN, FAD, CoA, etc are formed. Coenzymes are non-proteinaceous substance necessory for the activity of the enzymes. Proteins
(1) The word protein was coined by Berzelius in 1838 and was used by G. J. Mulder first time 1840.
(2) 15% of protoplasm is made up of protein. Average proteins contain 16% nitrogen, 50–55% carbon, oxygen 20–24%, hydrogen 7% and sulphur 0.3 – 0.5%. Iron, phosphorous, copper, calcium, and iodine are also present in small quantity.
(3) Structure of proteins: It is due to different rearrangement of amino acids. When carboxyl group of one amino acid binded with amino group (– NH2) of another amino acid the bond is called peptide bond. A peptide may be dipeptide, tripeptide and polypeptide. The simplest protein is Insulin. According to Sanger (1953) insulin consists of 51 amino acids. A protein can have up to four level of conformation.
(i) Primary structure: The primary structure is the covalent connections of a protein. It refers to linear sequence, number and nature of amino acids bonded together with peptide bonds only. e.g. ribonuclease, insulin, haemoglobin, etc.
(ii) Secondary structure: The folding of a linear polypeptide chain into specific coiled structure (α - helix) is called secondary structure and if it is with intermolecular hydrogen bonds the structure is known as ß - pleated sheet. α - helical structure is found in protein of fur, keratin of hair claws, and feathers. ß - pleated structure is found in silk fibres.
(iii) Tertiary structure: The arrangement and interconnection of proteins into specific loops and bends is called tertiary structure of proteins. It is stabilized by hydrogen bond, ionic bond, hydrophobic bond and disulphide bonds. It is found in myoglobin (globular proteins).
(iv) Quaternary structure: It is shown by protein containing more than one peptide chain. The protein consists of identical units. It is known as homologous quaternary structure e.g. lactic dehydrogenase. If the units are dissimilar, it is called as heterogeneous quaternary structure
e.g. hemoglobin which consists of two α - chains and two ß - chains.

