Read and download free pdf of CBSE Class 10 Chemistry Periodic Classification of Elements Worksheet Set D. Download printable Science Class 10 Worksheets in pdf format, CBSE Class 10 Science Chapter 5 Periodic Classification of Elements Worksheet has been prepared as per the latest syllabus and exam pattern issued by CBSE, NCERT and KVS. Also download free pdf Science Class 10 Assignments and practice them daily to get better marks in tests and exams for Class 10. Free chapter wise worksheets with answers have been designed by Class 10 teachers as per latest examination pattern
Chapter 5 Periodic Classification of Elements Science Worksheet for Class 10
Class 10 Science students should refer to the following printable worksheet in Pdf in Class 10. This test paper with questions and solutions for Class 10 Science will be very useful for tests and exams and help you to score better marks
Class 10 Science Chapter 5 Periodic Classification of Elements Worksheet Pdf
Question. Fill in the blanks
(a) The elements are arranged in such a way that.............. are on left side of zig-zag line and .............. on the right side.
(b) In the Mendeleev’s periodic table, properties of elements are periodic function of their ................ . periodic function of ................ .
Answer :
(a) Metals, non-metals, (b) Atomic masses, (c) Atomic number.
Case based Questions
Atoms of eight elements A, B, C, D, E, F, G and H have the same number of shells but different number of electrons in their outermost shell. It was found that elements A and G combine to form an ionic compound. This compound is added in a small amount to almost all vegetable dishes during cooking. Oxides of elements A and B are basic in nature while those of E and F are acidic. The oxide of D is almost neutral.
Based on the above information answer the following questions:
Question. To which group or period of the Periodic Table do the listed elements belong?
Answer. A and B belong to group 1 and 2 because they form basic oxides.
C belongs to group 13 as it has 3 valence electrons.
D belongs to group 14 as it forms almost neutral oxide.
E and F belong to group 15 and 16 as they form acidic oxides,
G belongs to group 17 as it has 7 valence electrons and
H belongs to group 18.
They belong to 3rd period of the Periodic Table because AG is NaCl, added in a small amount to almost all vegetable dishes during cooking and Na and Cl belong to 3rd period.
Question. What would be the nature of compound formed by a combination of elements B and F?
Answer. Ionic compounds will be formed because ‘B’ is metal and ‘F’ is non-metal. ‘B’ can lose two electrons and ‘F’ can gain two electrons.
Question. Which two of these elements could definitely be metals?
Answer. A and B are definitely metals as they form basic oxides.
Question. Which one of the eight elements is most likely to be found in gaseous state at room temperature?
Answer. G and H are gaseous at room temperature.
Question. If the number of electrons in the outermost shell of elements C and G be 3 and 7 respectively, write the formula of the compound formed by the combination of C and G.
Answer. CG3 is the formula of the compound formed by combination of C and G.
DESCRIPTIVE QUESTIONS
Question. What is the basis for arrangement of elements in the Mendeleev periodic table?
Answer. Increasing order of atomic mass and similarity in chemical properties
Question. State one reason for placing Mg and Ca in the same group of the periodic table.
Answer. They have same number of valence electrons and similar chemical properties.
Question. Name any three metalloids.
Answer. Boron, Silicon and Germanium.
Question. State the modern periodic law of classification of elements.
Answer. It states that “the properties of the elements are periodic functions of their atomic numbers.”
Question. Write the number of groups or vertical columns and periods or horizontal rows in the modern periodic table.
Answer. There are 18 vertical columns or groups and seven horizontal rows or periods in the modern periodic table.
Very Short Answers Type Questions
Question. Write the name, symbol and electronic configuration of an element X whose atomic number is 11.
Answer :
The element whose atomic number is 11 is sodium.
Its symbol is Na.
Electronic configuration – 2, 8, 1
Valency 1.
Question. Write the formulae of chlorides of Eka-silicon and Eka-aluminium.
Answer : GeCl4, GaCl3.
Question. What were the criteria used by Mendeleev in creating his periodic table ?
OR
State Mendeleev’s Periodic Law.
Answer :
Mendeleev’s periodic table was based on atomic masses and similarity in formula of hydrides and oxides of elements. According to him, the properties of elements are a periodic function of their atomic masses.
Question. In the Modern Periodic Table, Calcium (atomic number 20) is surrounded by elements with atomic numbers 12, 19, 21, and 38. Which of these have physical and chemical properties resembling calcium ?
Answer : The element with atomic number 12 and 38 has same chemical properties as that of Calcium because they have same number of valence electrons i.e., 2 electrons.
Question. How would the tendency to lose electrons change as we go from left to right across a period of the periodic table ?
Answer : On moving from left to right in a period, the tendency of atoms to lose electrons decreases.
Question. In the modern periodic table a zig-zag line separates metals from non-metals. What are these elements called and why ?
Answer : These are known as metalloids because they have some properties of metals and some properties of non-metals.
Question. Why do you think the noble gases are placed in a separate group ?
Answer : Noble gases are placed in a separate group because these are inert elements. They have properties which are different from all other elements.
Question. The following table shows elements represented by the letters A, B, C, D, E, F, G and H:
(i) Which of the element has the atomic size (a) biggest and (b) smallest?
(ii) Which element has valency (a) 3 and (b) Zero
Answer : (i) (a) ‘A’ is biggest in size (b) ‘G’ is smallest in size
(ii) (a) ‘C’ has valency 3. (b) H has zero valency
Question. An element ‘X’ belongs to 3rd period and group 16 of the modern periodic table.
(i) Determine the number of valence electrons and valency of ‘X’.
(ii) Molecular formula of the compound, when ‘X’ reacts with hydrogen and write its electron dot structure.
(iii) Name the element ‘X’ and state whether it is metallic or non-metallic.
Answer : (i) The element is S(16)—2, 8, 6, The number of valence electrons—6, Valency—2.
(iii) Sulphur, non-metallic
Question. What is a metalloid? Name any one of them.
Answer : The element which resembles both with metals and non-metals is called a metalloid, e.g. Boron, Silicon
Question. An element ‘M’ with electronic configuration (2, 8, 2) combines separately with NO3–, SO42– and PO43– radicals. Write the formulae of three compounds so formed. To which group and period of modern periodic table, ‘M’ belongs to? Will ‘M’ form covalent or ionic compounds? Give reason to justify your answer.
Answer : Mg(NO3)2, MgSO4, Mg3(PO4)2
It belongs to Group 2, 3rd period of the periodic table.
It will form ionic compounds because it can lose 2 electrons easily to form Mg2+ ions.
Question. What is place of metalloid in the periodic table?
Answer : They are placed between metals and non-metals in a zig-zag manner.
Question. Two elements ‘P’ and ‘Q’ belong to the same period of the modern periodic table and are in Group 1 and 2 respectively. Compare the following characteristics in tabular form:
(a) The number of valence electrons in their atom.
(b) Their metallic character (c) The size of their atoms
(d) The formulae of their oxides (e) Their tendency to lose electrons
(f) The formula of their chloride
Answer :
SHORT ANSWER TYPE QUESTIONS
Question. Use Mendeleev’s periodic table to predict the formulae for the oxides of the following elements : K, C, Al, Si, Ba.
Answer : K belongs to group 1. Therefore, the oxide will be K2O.
C belongs to group 4. Therefore, the oxide will be CO2.
Al belongs to group 3. Therefore, the oxide will be Al2O3.
Si belongs to group 4. Therefore, the oxide will be SiO2.
Ba belongs to group 2. Therefore, the oxide will be BaO.
Question. Na, Mg and Al are the elements of the 3rd period of the Modern Periodic Table having group number 1, 2 and 13 respectively. Which one of these elements has the (a) highest valency, (b) largest atomic radius, and (c) maximum chemical reactivity? Justify your answer stating the reason for each.
Answer :
(a) The element having the highest valency signifies the maximum number of electrons present in the valence shell of an atom. Hence, as per the given electronic configurations, the element having highest valency is aluminium (Al).
(b) As we move across the period, i.e., from left to right the atomic radius decreases. Therefore the element having the largest atomic radius will be sodium (Na).
(c) The given three elements are metals. So, the chemical reactivity of a metal is determined by its metallic character which is the tendency of an atom to lose its electrons. We know that the metallic character of element decreases across the period, i.e., from left to right. So, the element having highest chemical reactivity is sodium (Na)
Question. How it can be proved that the basic structure of the Modern Periodic Table is based on the electronic configuration of atoms of different elements ?
Answer : Modern periodic law states that the physical and chemical properties of an element are the periodic function of the atomic number of that element. Electronic configuration of the elements play an important role in the placement of element in the modern periodic table. The valence shell electron of an element decides its position in a particular group or period for example : if the configuration of an element is 2, 1 it means that the Li = 2, 1 It belongs to the 2nd period and 1st group.
Question. Can the following groups of elements be classified as Dobereiner’s triad :
(a) Na, Si, Cl (b) Be, Mg, Ca
Atomic mass of Be-9; Na-23, Mg-24, Si-28, Cl-35, Ca-40, Justify your answer in each case.
Answer : (a) Na, Si, Cl cannot be classified as Dobereiner’s triad because here the elements do not belong to the same group and have different electronic configuration.
Na– 2, 8, 1; Si – 2, 8, 4 and Cl – 2, 8, 7
(b) Be, Mg, Ca are the elements of Dobereiner‘s triad because the mass of Mg is the arithmetic mean of the other two elements i.e., Be and Ca = (9 + 40)/2
= 24.5.
Question. Write the number of periods and groups in the Modern Periodic Table. How does the metallic character of elements vary on moving (a) from left to right in a period and (b) down a group ? Give reason to justify your answer.
Answer : In the Modern Periodic Table, there are 18 vertical columns known as Groups and 7 horizontal rows known as Periods.
Metallic character : It is defined as the tendency of an atom to lose electrons.
(a) Across the period i.e., from left to right, metallic character decreases.
(b) Down the group i.e., from top to bottom, metallic character increases.
Reason : Across the period, the effective nuclear charge increases, thus decreasing its atomic radius. This favours the electronegativity and therefore the tendency to lose electrons is low. This accounts for the decrease in the metallic character. As we move down the group, the number of shells keep on increasing and therefore the atomic size increases and electronegativity decreases. This enhances the ability to lose electrons and therefore the metallic character increases.
Question. Two elements X and Y have atomic numbers 12 and 16 respectively. To which period of the modern periodic table do these two elements belong? What type of bond will be formed between them and why? Also give the chemical formula of the compound formed.
Answer : Electronic configuration of X: 2,8,2, Y: 2,8,6
Both X and Y belong to 3rd period because they have 3 shells.
Ionic bond will be formed.
Reason: X will lose 2 electrons and Y will gain 2 electrons to complete their octet and become stable.
Formula is
Question. Na, Mg and Al are the elements of the 3rd period of the Modern Periodic Table having group number 1, 2 and 13 respectively. Which one of these elements has the (a) highest valency, (b) largest atomic radius and
(c) maximum chemical reactivity ? Justify your answer stating the reason for each.
Answer : Sodium (Na), At. number 11, 2, 8, 1
Magnesium (Mg), At. number 12, 2, 8, 2
Aluminium (Al), At. number 13, 2, 8, 3
(a) The element having the highest valency is Al, as it has 3 valence electrons.
(b) The element with the largest atomic radius is Na as left to right atomic radius decreases.
(c) The element with maximum chemical reactivity is Na as metallic character decreases left to right.
Question. What is periodicity in properties of elements with reference to the Modern Periodic Table ? Why do all the elements of the same group have similar properties ? How does the tendency of elements to gain electrons change as we move from left to right in a period ? State the reason of this change.
Answer : The occurrence of the elements with similar properties after certain regular intervals when they are arranged in increasing order of atomic number is called periodicity. The periodic repetition of the properties is due to the recurrence of similar valence shell configuration after regular interval.
The elements in a group have same valence electrons thus similar chemical properties. In a period, tendency to gain electrons increases from left to right. This is due to increase in effective nuclear change and decrease in atomic radius.
Question. Based on the group valency of elements write the molecular formula of the following compounds giving justification for each
(i) Oxides of first group elements
(ii) Halides of group 13 and
(iii) Compound formed when an element A of group 2 combines with element B of group 17
Answer : (i) Group 1 elements have valency equal to 1.
Question. An element P (atomic number 20) reacts with an element Q (atomic number 17) to form a compound.
Answer the following questions giving reason :
Write the position of P and Q in the Modern Periodic Table and the molecular formula of the compound formed when P reacts with Q.
Answer :
P = 20 : 2, 8, 8, 2
Q = 17 : 2, 8, 7
P = Period 4 and Group 2
Q = Period 3 and Group 17
Hence, formula of the compound formed between P and Q is PQ2.
Question. Name two elements you would expect to show chemical reactions similar to magnesium. What is the basis for your choice ?
Answer : Calcium (Ca) and strontium (Sr) are expected to show chemical reactions similar to magnesium (Mg). This is because the number of valence electrons (2) is same in all these three elements and they belong to same group.
Question. (a) What property do all elements in the same column of the periodic table as boron have in common ?
(b) What property do all elements in the same column of the periodic table as fluorine have in common ?
Answer :
(a) All the elements in the same column as boron have the same number of valence electrons i.e., 3. Hence, they all have valency equal to 3.
(b) All the elements in the same column as fluorine have the same number of valence electrons i.e., 7.
Hence, they all have valency equal to 1.
Question. An element X, which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants.
(i) Identify the element X.
(ii) Write the electronic configuration of X.
(iii) Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals.
(iv) What would be the nature (acidic/basic) of the oxides formed?
(v) Locate the position of the element in the modern periodic table
Answer :
Question. Based on the group valency of elements, state the formula of the following, giving justification for each.
(i) Oxides of Group 1 elements.
(ii) Halides of the elements of Group 13.
(iii) Compounds formed when an element of group 2 combines with an element of Group 16.
Answer : (i) Group 1 elements can lose one electron to become stable, so its valency is equal to 1, M2O.
Question. The atomic number of Na and Mg is 11 and 12 respectively and they belong to same period.
(a) Which one would have smaller atomic size?
(b) Which one would be more electropositive?
(c) To which group would each one belongs?
Answer : (a) Magnesium has smaller size than Na.
(b) Na is more electropositive than Mg.
(c) Na belongs to Group 1, Mg belongs to Group 2.
Question. The elements Be, Mg and Ca are having two electrons in their outermost shells are in periods 2, 3 and 4, respectively of the modern periodic table. Answer the following questions, giving justification in each case.
(i) Write the group to which these elements belong.
(ii) Name the least reactive element.
(iii) Name the element having largest radius.
Answer : (i) They belong to Group 2 because they have 2 valence electrons.
(ii) Be is the least reactive element due to smallest size and least tendency to lose electrons.
(iii) Ca has largest radius because it has the most, four shells (2, 8, 8, 2).
Question. An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
(a) Where in the periodic table are elements X and Y placed ?
(b) Classify X and Y as metal (s), non-metal (s) or metalloid (s).
(c) What will be the nature of oxide of element Y ? Identify the nature of bonding in the compound formed.
Answer : (a) X belongs to Group 17 and 3rd period Y belongs to Group 2 and 4th period.
(b) X — Non-metal and Y — Metal.
(c) Basic oxide; Ionic bonding.
Question. An element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants.
(a) Identify the element X.
(b) Write the electronic configuration of X.
(c) Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals.
(d) What would be the nature (acidic or basic) of oxides formed ?
(e) Locate the position of the element in the modern periodic table.
Answer : (a) Element X is sulphur (atomic no. 16).
(b) K, L, M
2 8 6
(c) 2 FeSO4 (s) Heat→ Fe2O3(s) + SO2 (g) + SO3 (g)
(d) Acidic
(e) 3rd period, group 16
Question. An element has same number of electrons in the first and the fourth shell as well as in the second and the third shell.
(a) Write down the electronic configuration of the element.
(b) Write down group number and period to which it belongs.
(c) What is the valency of the element ?
Answer : (a) The electronic configuration of the element is 2, 8,8, 2. The element is calcium.
(b) Period 4 and group 2.
(c) Valency of element is 2.
Question. (a). On moving from left to right in the second period when happens to the number of valence electrons?
(b). How does reactivity of metals vary down a group?
Answer. a. Number of valence electrons increases from left to right in the second period
b. Reactivity of metals goes on increasing down a group.
Question. The elements of the second period of the Periodic Table are given below: Li Be B C N O F
(a) Give reason to explain why atomic radii decrease from Li to F.
(b) Identify the most (i) metallic and (ii)non-metallic element.
Answer. (a) It is because nuclear charge increases due to increase in atomic number, therefore, force of Attraction between nucleus and valence electrons increases, i.e. effective nuclear charge increases, hence atomic radii decrease from Li to F.
(b) (i) Most metallic element is ‘Li’ as it can lose electrons easily due to larger atomic size.
(ii) Most non-metallic element is ‘F’ because it can gain electrons easily due to smallest atomic size.
Question. The elements of the third period of the Periodic Table are given below:
Na, Mg, Al, Si, P,S, Cl, Ar
(a) Which atom is bigger, Na or Mg? Why?
(b) Identify the most (i) metallic and (ii) non-metallic element in Period 3.
Answer. (a) Sodium is bigger than magnesium as it has lesser nuclear charge so there is less force of attraction between nucleus and valence electrons and less effective nuclear charge. It is, therefore, bigger in size.
(b) (i) Sodium is the most metallic as it can lose electrons easily due to its larger atomic size,
(ii) Chlorine is the most non-metallic element because it can gain electrons easily due to its
smallest atomic size.
Question. Mention three achievements of Mendeleev’s periodic table and one defect.
Answer. Merits (a) It could classify all the elements discovered at that time.
(b) It helped in discovery of new elements.
(c) It helped in correction of atomic mass of some of the elements.
Defect;1. All isotopes of an element do not find position in this table.
Question. How can the valency of an element be determined if its electronic configuration is known? What will be the valency of an element of atomic number 9 (nine)?
Answer. It is the combining capacity of the element.
Generally, valency=number of valence electron or 8 - number of valence electron.
If the element has 1, 2, 3, 4 valence electrons, its valency will be 1, 2, 3, 4 respectively.
If the element has 5, 6, 7, 8 valence electrons, its valency will be 3, 2, 1, 0.
Element with atomic number 9 has electronic configuration 2, 7. So, its valency will be 1.
SHORT ANSWER TYPE QUESTIONS
Question. (a). How does the electronic configuration of an atom of an element related to its position in the modern periodic table? Explain with one example.
(b)Find the group and period of element with Z=12?
Answer. (a)The position of element depends upon number of valence electrons which depend upon electronic configuration. Those elements which have same valence electrons, occupy same group. Eg; those elements which have one valence electron belong to group 1. Elements with two valence electrons belong to group 2. Period number is equal to number of shells. If valence electrons are equal to 1, it belongs to group 1. If it has 2 shells, it belongs to second period, e.g. if element ‘X’ has atomic number 11, its electronic configuration is 2, 8,1. It has one valence electron, it belongs to group 1 and it has three shells therefore, it is in third period.
(b)Z=12and the configuration is 2,8,2So it should belong to 2 nd group and 3 rd period
Question. The atomic numbers of three elements, X, Y and Z are 9,11 and 17 respectively. Which two of these elements will show similar chemical properties? Why?
Answer. Electronic configuration of X, Y and Z will be: X (9) : 2, 7 Y(11) : 2, 8, 1 Z(17) : 2, 8, 7
X and Z will show similar chemical properties due to same number of valence electrons.
Question. How does the metallic character of elements change along a period of the periodic table from the left to the right and in a group from top to bottom .why?
Answer. The metallic character goes on decreasing along a period from left to right because atomic size goes on decreasing therefore, tendency to lose electrons decreases. But in a group from top to bottom metallic character goes on increasing due to increase in size from top to bottom giving a tendency to lose electrons which are loosely held.
Question. How does the valency of elements vary
(a) in going down a group, and
(b) in going from left to right in a period of the periodic table
Answer. (a) Valency remains the same in a group.
(b) Valency first goes on increasing from left to right in a period till middle of period, then
Decreases
Question. (a) In the Mendeleev’s Periodic table, why does Argon with atomic mass 39.9 appear before Potassium having atomic mass 39.1?
(b) Why is atomic number a more important property than atomic mass?
Answer. (a).It is because Mendeleev arranged elements giving more importance to the similarity in chemical properties of an element with the remaining members of the group .So Argon ,being an inert gas was placed along with inert gases and Potassium being an alkali metal is placed along with the other alkali metals. So Argon and Potassium are examples of anomalous pair
(b)Atomic number gives us the electronic configuration which gives the number of valence electron which decides the chemical nature of the element.
5 MARK QUESTIONS
Question. (a) What is a group in the periodic table ? In which part of a group would you separately expect the elements to have : (i) the greatest metallic character, (ii) the largest atomic size ?
(b) In what respects do the properties of group 1 elements differ from those of group 17 elements ?
(c) From the stand point of atomic structure, what determines which element will be the first and which is the periodic table ?
(d) Explain why, the properties of elements are repeated after 2, 8, 18 and 32 elements in the periodic table.
(e) What are the advantages of the periodic table ?
Answer : (a) The vertical columns in a periodic table are called groups.
(i) The greatest metallic character is found in the elements in the lowest part of the group.
(ii) The largest atomic size is found in the lowest part of the group.
(b) Group 1 elements have 1 valence electron and are ionic in chemical reactions, whereas, the elements of group 17 have 7 valence electrons. They all are non-metals.
(c) The number of valence electrons is the atoms of elements decides which element in a period and which will be the last in a period.
(d) The properties of elements are repeated after 2, 8, 18 and 32 elements in the periodic table because the electronic configurations of the elements are repeated in this manner.
(e) Advantages of the periodic table :
(i) It is easier to remember the properties of an element if its position in the periodic table is known.
(ii) The type of compounds formed by an element can be predicted by knowing its position in the periodic table.
Question. Explain giving justification the trends in the following properties of elements, on moving from left to right in a period in the Modern periodic Table.
(a) Variation of valency.
(b) Change of atomic radius.
(c) Metallic to non-metallic character.
(d) Electronegative character.
(e) Nature of oxides.
Answer : (a) Valency first increases, then decreases on moving from left to right in a period.
(b) Atomic radius decreases from left to right in a period.
(c) Metallic to non-metallic character increases from left to right in a period.
(d) Electronegative character increases from left to right in period.
(e) Nature of oxides changes from basic to acidic on moving from left to right in a period.
Question. a. Calcium is an element with atomic number 20. (i) Is it a metal or non-metal? (ii) Will its size be more or smaller than that of potassium? (iii) Write the formula of its chloride. b. An element ‘X’ has mass number 35 and number of neutrons 18. Write atomic number and electronic configuration of ‘X’. Also write group number, period number and valency of ‘X’.
Answer. a.The electronic configuration of calcium (Z = 20) is 2, 8, 8, 2. (i) Since it has only two valence electrons, it is present in group 2. It is a metal. (ii) Both potassium (K) and calcium (Ca) are present in fourth period. Since atomic size decreases along a period, calcium is smaller in size. (iii) The valency of calcium is 2. The formula of its chloride is CaCl2.
b. Atomic number of the element ‘X’ = 35 – 18 = 17
Gp17, period-3
Question.Two elements X and Y belong to group 1 and 2 respectively in the same period of periodic table. Compare them with respect to: (i) the number of valence electrons in their atoms; (ii) their valencies; (iii) metallic character; (iv) the sizes of their atoms; (v) the formulae of their oxides; (vi) the formulae of their chlorides.
Answer. X and Y belong to same period, X belongs to group ‘1’. Y belongs to group ‘2’. (i) Valence electron in X is 1 whereas valence electrons in Y are 2. (ii) The valency of X is 1 whereas valency of Y is 2. (iii) X is more metallic than Y because metallic character decreases on moving from left to right in a period. (iv) The size of X is more than Y because size of the atom decreases on moving from left to right in a period. (v) Oxide of X = X2O, Oxide of Y = YO (vi) Chloride of X = XCl, Chloride of Y = YCl2
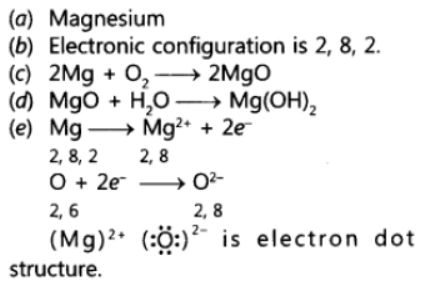
Question. An element is placed in 2nd Group and 3rd Period of the Periodic Table, burns in presence of oxygen to form a basic oxide.
(a) Identify the element
(b) Write the electronic configuration
(c) Write the balanced equation when it burns in the presence of air
(d) Write a balanced equation when this oxide is dissolved in water
(e) Draw the electron dot structure for the formation of this oxide
Answer.
Question. An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
(a) Where in the periodic table are elements X and Y placed?
(b) Classify X and Y as metal (s), non-metal (s) or metalloid (s)
(c) What will be the nature of oxide of element Y? Identify the nature of bonding in the compound formed
(d) Draw the electron dot structure of the divalent halide
Answer.

1 Mark Questions
1. Arrange the following in decreasing atomic size:
(1) Na , Mg , K (2) N , F ,O (3) N , S , P
2. Give the name and electronic configuration of the second alkali metal belonging to the third period.
3. What is common in the elements belonging to the same period in the periodic table?
4. State modern periodic law of classification of elements.
5. Write two reasons responsible for the late discovery of noble gases.
2Marks Questions
6. How does metallic character of the elements vary
(a) down a group (b) across a period ?
7. Element Y with atomic number 3 combines with an element A having atomic number 17 .What would be the formula of the compound?
8. Define the terms: (1) atomic radius (2) valency
9. What would be the nature of the oxides formed by the elements on :
(a) the left hand side of the periodic table
(b) the right hand side of the periodic table?
10. State Newland’s Law of Octaves. Mention any two limitations.
3 Marks Questions
11. The atomic number of elements A ,B , C , D and E are given below;
From the above table ,answer the following questions;
(a) Which two elements are chemically similar?
(b)Which is an inert gas?
(c) Which element belongs to third period of the periodic table?
(d)Which element among these is a non-metal?
12. Na , Mg and Al are the elements having 1,2,3 electrons respectively in the outer most shell. Which of the elements:
(a) has the largest atomic radius?
(b) least reactive?
Justify your answer.
13. What are the achievements of Mendeleev’s Periodic table?
14. The atomic number of Cl is 17. On the basis of this information, answer the questions that follow:
(a) Write the electronic configuration of Cl.
(b) Find its valency.
(c) To which group and period does it belong?
5 Marks Questions
15. The position of elements A, B, C, D, E, F and G in the Modern Periodic Table is given as under:
(a) In which group are inert elements placed?
(b)What type of ions would B, C, E and F form?
(c) How many shells would A have?
(d)What is the similarity between A and D?
(e) Identify the most abundant element in the earth’s crust.
16. Given below are some elements of the modern periodic table:
4Be,9F,14Si,19K,20Ca
(a) Select the element that has one electron in the outermost shell. Write its electronic configuration.
(b) Select two elements that belong to the same group. Give reason for your answer.
(c) Select two elements that belong to the same period. Which one of the two has bigger atomic size?
17. Compare and contrast the arrangement of elements in Mendeleev’s and the Modern periodic table.
Please click on below link to download CBSE Class 10 Chemistry Periodic Classification of Elements Worksheet Set D
| CBSE Class 10 Biology Our Environment Worksheet Set A |
| CBSE Class 10 Biology Our Environment Worksheet Set B |
| CBSE Class 10 Biology Our Environment Worksheet Set C |
Chapter 5 Periodic Classification of Elements CBSE Class 10 Science Worksheet
The above practice worksheet for Chapter 5 Periodic Classification of Elements has been designed as per the current syllabus for Class 10 Science released by CBSE. Students studying in Class 10 can easily download in Pdf format and practice the questions and answers given in the above practice worksheet for Class 10 Science on a daily basis. All the latest practice worksheets with solutions have been developed for Science by referring to the most important and regularly asked topics that the students should learn and practice to get better scores in their examinations. Studiestoday is the best portal for Printable Worksheets for Class 10 Science students to get all the latest study material free of cost. Teachers of studiestoday have referred to the NCERT book for Class 10 Science to develop the Science Class 10 worksheet. After solving the questions given in the practice sheet which have been developed as per the latest course books also refer to the NCERT solutions for Class 10 Science designed by our teachers. After solving these you should also refer to Class 10 Science MCQ Test for the same chapter. We have also provided a lot of other Worksheets for Class 10 Science which you can use to further make yourself better in Science.
You can download the CBSE Practice worksheets for Class 10 Science Chapter 5 Periodic Classification of Elements for the latest session from StudiesToday.com
Yes, the Practice worksheets issued for Chapter 5 Periodic Classification of Elements Class 10 Science have been made available here for the latest academic session
There is no charge for the Practice worksheets for Class 10 CBSE Science Chapter 5 Periodic Classification of Elements you can download everything free
Regular revision of practice worksheets given on studiestoday for Class 10 subject Science Chapter 5 Periodic Classification of Elements can help you to score better marks in exams
Yes, studiestoday.com provides all the latest Class 10 Science Chapter 5 Periodic Classification of Elements test practice sheets with answers based on the latest books for the current academic session

