Access the latest CBSE Class 10 Chemistry Carbon And Its Compounds Worksheet Set G. We have provided free printable Class 10 Science worksheets in PDF format, specifically designed for Chapter 04 Carbon and Its Compounds. These practice sets are prepared by expert teachers following the 2025-26 syllabus and exam patterns issued by CBSE, NCERT, and KVS.
Chapter 04 Carbon and Its Compounds Science Practice Worksheet for Class 10
Students should use these Class 10 Science chapter-wise worksheets for daily practice to improve their conceptual understanding. This detailed test papers include important questions and solutions for Chapter 04 Carbon and Its Compounds, to help you prepare for school tests and final examination. Regular practice of these Class 10 Science questions will help improve your problem-solving speed and exam accuracy for the 2026 session.
Download Class 10 Science Chapter 04 Carbon and Its Compounds Worksheet PDF
Question. Glacial acetic acid is a
(a) Frozen acetic acid
(b) 5-8% of solution of acetic acid in water
(c) Mixture of acetic acid and alcohol
(d) None of these
Answer: B
Question. The chemical reaction shows the addition of chlorine gas to hydrocarbon in the presence of sunlight.
CHCl3 + Cl2 → CCl4 + HCl
How does chlorine react to a hydrocarbon compound in the presence of sunlight?
(a) it adds hydrogen into the compound
(b) it adds an oxygen atom into the compound
(c) it substitutes hydrogen atom from the compound
(d) it breaks double and triple bonds into a single bond
Answer: C
Question. The image represents the structure of a few hydrocarbon compounds.
Which of these compounds can be classified as alkynes?
(a) only (A)
(b) only (B)
(c) both (A) and (D)
(d) both (B) and (C)
Answer: C
Question. The electronic configuration of an element is found to be 2, 4. How many bonds can one carbon atom form in a compound?
(a) 1
(b) 2
(c) 4
(d) 6
Answer: C
Question. Oils on treating with hydrogen in the presence of palladium or nickel catalyst forms fats. This is an example of
(a) addition reaction
(b) substitution reaction
(c) displacement reaction
(d) oxidation reaction
Answer: A
Question. The gas evolved when ethanol reacts with sodium metal is
(a) H2
(b) CO2
(c) H2O
(d) CO
Answer: A
Question. Methane, ethane and propane are said to form a homologous series because all are-
(a) Hydrocarbons
(b) saturated compounds
(c) aliphatic compounds
(d) differ from each other by a CH2 group
Answer: D
Question. Which of the following is incorrectly matched?
(a) Vinegar → carboxylic acid
(b) C2H6 → alkane
(c) Ethanol → alcohol
(d) Methanol → ketone
Answer: D
Question. Which of the following belongs to a homologous series of alkynes?
C6H6, C2H6, C2H4, C3H4
(a) C6H6
(b) C2H6
(c) C2H4
(d) C3H4
Answer: D
Question. The carbon exist in the atmosphere in the form of
(a) Carbon monoxide only
(b) Carbon monoxide in traces and carbon dioxide
(c) carbon dioxide only
(d) coal
Answer: B
Question. Pentane with molecular formula C5H12 has
(a) 12 covalent bonds
(b) 16 covalent bonds
(c) 18 covalent bonds
(d) 15 covalent bonds
Answer : B
Question. Which of the following statements is incorrect regarding a homologous series?
(i) Compounds in a homologous series can have the same or different functional group.
(ii) Compounds in a homologous series have very less similarity in chemical properties.
(iii) Difference between the two successive compounds in a homologous series differ by a CH2 group.
(iv) Successive members in a homologous series differ in molecular mass by 14 units
(a) i and ii
(b) ii and iii
(c) iii and iv
(d) i and iv
Answer : A
Question. Which of the following aliphatic compounds, is saturated molecule?
(a) C6H12
(b) C2H2
(c) C5H10
(d) C4H10
Answer : D
Question. Three of the four compounds belong to a homologous series. Identify the odd one out.
(a) C4H10
(b) C2H4
(c) C3H8
(d) C5H12
Answer : B
Question. Which of these is not a property of Carbon?
(a) Catenation
(b) Tetravalency
(c) Formation of ionic bonds
(d) Tendency to form multiple bonds
Answer : C
VERY SHORT ANSWER TYPE QUESTIONS:
Question. Write the molecular formula of an alkyne containing 10 atoms of hydrogen.
Answer: C6H10.
Question. What is the function of conc. H2SO4 in the formation of ethene from ethanol?
Answer: Dehydrating agent
Question. Identify the functional group present in the following compound:
Answer: Aldehyde.
Question. Write the name and formula of the 2nd member of homologous series having general formula CnH2n
Answer: CnH2n : Alkene , 2nd member = C3H6 (propene)
SHORT ANSWER TYPE QUESTIONS:
Question. What are covalent bonds? Show their formation with the help of electron dot structure of . Why are covalent compounds generally poor conductors of electricity?
Answer: Covalent bonds are those bonds which are formed by sharing of the valence electrons between two atoms. Electron dot structure of methane is shown in the figure.
Covalent compounds are generally poor conductors of electricity because they do not have tree electrons or ions.
Question. Covalent compounds have low melting and boiling point. Why?
Answer: Covalent compounds have low melting and boiling points because the forces of attraction between molecules of covalent compounds are very weak. On applying a small amount of heat these molecular forces break.
3. Write the name and structure of an aldehyde with 4 carbon atoms.
Answer: C3H7 CHO (Butanal)
4. Draw the electron dot structure of ethane (C2H6).
Answer:
5. Draw the structure of pentanal (C4H9CHO).
Answer:
6. Name the functional group present in each of the following compounds.
Answer: C3H7OH — Alcoholic group
7. Draw the structure of ethene molecule (C2H4).
Answer:
Question. Write the next homologue of each of the following:
(i) C2H4
(ii) C4H6
Answer: (i) C2H4 belongs to alkene series having general formula, CnH2n.
Thus, next homologue will be C3H2×3 = C3H6
(ii) C4H6 belongs to alkyne series having general formula, CnH2n-2.
Thus, next homologue will be C5H2×5-2 = C5H8
Question. What are covalent compounds? Why are they different from ionic compounds? List their three characteristic properties.
Answer: Covalent compounds are those compounds which are formed by sharing of valence electrons between the atoms e.g., hydrogen molecule is formed by mutual sharing of electrons between two hydrogen atoms.
They are different from ionic compounds as ionic compounds are formed by the complete transfer of electrons from one atom to another e.g., NaCl is formed when one valence electron of sodium gets completely transferred to outer shell of chlorine atom. The characteristic properties of covalent compounds are:
(i) They are generally insoluble or less soluble in water but soluble in organic solvents.
(ii) They have low melting and boiling points.
(iii) They do not conduct electricity as they do not contain ions.
Question. Name the following compounds:
(a) CH3 – CH2 – OH
Answer: (a) CH3 – CH2 – OH : Ethanol
Question. Write the name and structure of an alcohol with three carbon atoms in its molecule.
Answer: An alcohol with three carbon atoms in its molecule is propanol. The structure of propanol is.
Question. Give reasons for the following:
(i) Element carbon forms compounds mainly by covalent bonding.
(ii) Diamond has high melting point.
(iii) Graphite is a good conductor of electricity.
Answer: (i) As carbon has four valence electrons and it can neither lose nor gain four electrons thus, it attains noble gas configuration only by sharing of electrons. Thus, it forms covalent compounds.
(ii) In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid threedimensional structure. This makes diamond the hardest known substance. Thus, it has high melting point.
(iii) In graphite, each carbon atom is bonded to three other carbon atoms by covalent bonds in the same plane giving a hexagonal array. Thus, only three valence electrons are used for bond formation and hence, the fourth valence electron is free to move. As a result, graphite is a good conductor of electricity.
Question. An element of group 14 has two common allotropes, A and B.
A is very hard and is bad conductor of electricity while B is soft to touch and good conductor of electricity. Identify the element and its allotropes.
Answer : Element is carbon
A is Diamond.
B is graphite
Question. Allotropes of carbon has same chemical properties. Give reason.
Answer : Chemical properties of an element depends on valence electrons. Allotropes have same number of valence electrons, hence same chemical properties.
Question. How many non-bonded electrons are there in?
a) Ammonia b) Methane c) Nitrogen
Answer : a) two electrons(1 pair) b) 0 c) 4(two pairs)
Question. Alkenes and alkynes are unsaturated. What does it mean?
Answer : Unsaturated molecules have carbon carbon double bonds or triple bonds.Alkenes and alkynes contain double or triple bond between carbon atoms, hence they are unsaturated. Unsaturated molecules are those in which more atoms can be added.(They undergo addition reaction)
Question. List any two properties of homologous series.
Answer : i) They show gradation in physical properties.
ii) Similarity in chemical properties
iii) They can be represented by a general formula
(any two properties can be written)
Question. Compare the catenation ability of Carbon and Silicon.
Answer : Carbon and Silicon, both with valency 4, have the ability to form covalent bonds by sharing of electrons. Both show the ability of self- combination- catenation.
In Carbon, the small size of carbon atoms, as also the formation of strong bonds by Carbon atoms among themselves and with atoms of other elements, makes carbon compounds very stable.
Silicon can form compounds having Si chains of up to 7 or 8 atoms. But due to weak bonds, these compounds are not stable.
Question. Atom of an element contains 5 electrons in the valence shell. This element exists as diatomic molecules, and is a major component of air.
(a) Identify the element.
(b) Show the bond formation between two atoms of this element.
(c) What is the nature of bond formed between the 2 atoms.
Answer : (a) Nitrogen
(b)
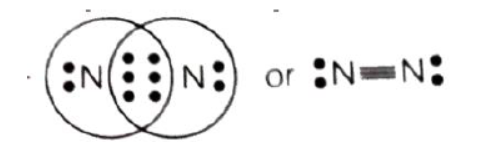
(c) Covalent bond
Question. An element X found in nature in solid form has 4 electrons in valence shell of its atom. Its allotrope Y has properties that allows it to be used as a dry lubricant, as also as a part of pencil lead.
(a) Identify the element.
(b) What is this allotrope Y?
(c) Write any 1 other use of this allotrope other than those mentioned here.
(d) Predict the ability of this allotrope to conduct electricity. Give reason.
(e) Name two other allotropes of this element other than Y.
Answer : (a) Carbon
(b) Graphite
(c) Used for making electrodes in dry cells.
(d) It is a good conductor of electricity.
Reason: In graphite, each carbon atom is covalently bonded with three other carbon atoms. So, only 3 valence electrons are used in bond formation and the 4th valence electron is free to move. Due to the presence of these free electrons (1 per carbon atom), graphite is a good conductor of electricity.
(e) Diamond, Buckminsterfullerene.
LONG ANSWER TYPE QUESTION:
Question. Two elements A and B have the property C by which they can combine with more atoms of their same type. Element A is a component of the gas D that is a respiratory byproduct, while element B is the second most abundant element in the crust.
(a) Identify the elements A and B.
(b)What is the property C?
(c) Identify the gas D.
(d) Among A and B, which one shows the property C to a greater extent? Why?
Answer : (a) A is Carbon; B is Silicon.
(b) Catenation.
(c) Carbon dioxide.
(d) A (Carbon) shows greater extent of catenation than B (Silicon)
Reason: Carbon atoms are smaller than that of silicon. So, carbon- carbon bonds are much stronger than silicon- silicon bonds.
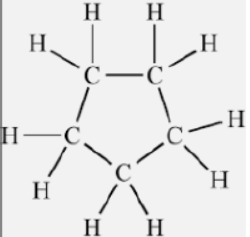
Question. A and B are two organic compounds with the same molecular formula C5H10.
Write their names and structural formulae in case
(a) A is a cyclic compound.
(b) B is a straight chain compound.
(c) Among A and B, which one will have only single bonds?
(d) Will it be A or B that has both single and double bonds?
Answer : (a) A is Cyclopentane.
(b) B is Pentene.
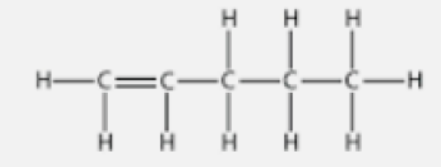
(Note: the double bond can be between any 2 of the 5 carbon atoms)
(c) A (Cyclopentane).
(d) B (Pentene)
Question. In the electron dot structure of hydrogen molecules, each individual atom is not satisfying the octet. Justify.
Answer : For hydrogen atom as there is only a K shell,it can occupy a maximum of two electrons.
Question. How many saturated hydrocarbons can be made using three carbon atoms? and hydrogen atoms? Name them.
Answer : Two.
Propane and cyclopropane.
Question. Carbon cannot make ionic compounds. Why?
Answer : Due to small size and high effective nuclear charge, carbon cannot lose electrons to form C4+ ion and as carbon with 6 protons cannot afford four more electrons in its L shell, it cannot form C4- ions, As carbon cannot form an anion or cation, it cannot make ionic bonds.
Question. Give the general formula of alkanes. Write the name, structural formula and physical state of the compound containing:
(i) 3-carbon atoms
(ii) 8-carbon atoms.
Answer : General formula of alkanes is CnH2n+2 where n = 1, 2, 3…
(i) Propane, CH3—CH2—CH3 Propane is a gas.
(ii) CH3—CH2—CH2—CH2—CH2—CH2—CH2—CH3 Octane is a liquid
Question. Why does carbon form compounds mainly by covalent bonding?
Answer : Carbon atoms have 4 valence electrons in their valence shell, it needs to gain or lose 4 electrons to attain the noble gas configuration.
(i) It could gain four electrons forming C4- anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons.
(ii) It could lose four electrons forming C4+ cation. But it would require a large amount of energy to remove four electrons from its outermost shell. Therefore, carbon shares its valence electrons to complete its octet with other atoms to form covalent bonds.
Question. List the common physical properties of carbon compounds.
Answer : They have covalent bonds between their atoms therefore they do not form ions. So they are poor conductors of electric current. These compounds have low melting and low boiling points. They are generally insoluble in water but soluble in the organic solvents like ether, carbon- tetrachloride, etc.
Question. Compare the structures of diamond and graphite.
Answer : In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three dimensional structure.
In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One of these bonds is a double bond.
Question. Write the general IUPAC names of alcohol, carboxylic acid, aldehyde and 2M ketone.
Answer : Compound General IUPAC name
Alcohol alkanol
Carboxylic acid Alkanoic acid
Aldehyde Alkanal
Ketone Alkanone
Question. Draw the electron dot structure of ethyne and also draw its structural formula.
Answer :
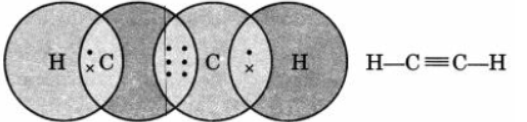
Question. Draw the electron dot structure of O2 and N2 molecules
Answer :
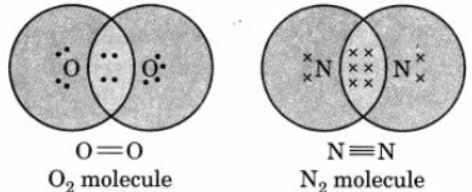
Question. State the reason why carbon can neither form C+4 cation nor C-4 anions but forms covalent compound.
Answer: Carbon has atomic number 6. This means that it has 4 electrons in its outermost shell. It needs to gain or lose 4 electrons to achieve noble gas configuration. But it cannot form C+4 cation because the removal of 4 electrons requires a large amount of energy. And also, cannot form C-4 anion as it would be difficult for its nucleus with 6 protons to hold on to 10 electrons. Therefore, Carbon atoms share electrons and form covalent compounds.
Question. The element carbon forms a very large number of compounds. Give reason for this fact.
Answer: Carbon forms large number of compounds because of tetravalency and catenation property.
Tetravalency- Carbon has valency 4 to attain noble gas configuration carbon shares its valence electrons with other elements like hydrogen chlorine etc.
Catenation-Carbon also shows the property of self-linking in which it forms long branched or cyclic chains to form large number of compounds.
Question. Why carbon and its compounds are used as fuels for most applications?
Answer: Carbon and its compounds give large amount of heat on combustion due to high percentage of carbon and hydrogen. They have high optimum ignition temperature with high calorific values and are easy to handle and their combustion can be controlled. Therefore, carbon and its compounds are used as fuels.
Question. What is a homologous series? List any of its two features.
Answer: A homologous series is a series of carbon compounds that have different numbers of carbon atoms but contain the same functional group.
Characteristics of homologous series:
1. The members of the homologous series can be represented by a general formula.
2. The successive members differ from each other in the molecular formula by a CH₂ unit.
Question. What is meant by isomers? Draw the structures of two isomers of butane. Explain why we cannot have isomers of first three members of alkane series?
Answer: Isomers are compounds having the same molecular formula but different structures. Since branching is not possible, isomers are not possible for the first three members of alkanes series.
The two isomers of butane, C4H10 are:
Question. List Any three difference between soap and detergent.
Answer:
| soap | detergent |
| i) Soaps are sodium salts of fatty acids. |
Detergents are sodium salt of sulphonic acids. |
| ii) Soaps clean well in soft water but do not clean well in hard water. | detergents clean well with both with hard and soft water |
| Soaps are biodegradable and do not cause pollution. |
Some detergents are nonbiodegradable and cause of pollution |
I) MCQs
Please click on below link to download CBSE Class 10 Chemistry Carbon And Its Compounds Worksheet Set G
| CBSE Class 10 Biology Our Environment Worksheet Set A |
| CBSE Class 10 Biology Our Environment Worksheet Set B |
| CBSE Class 10 Biology Our Environment Worksheet Set C |
Important Practice Resources for Class 10 Science
Chapter 04 Carbon and Its Compounds CBSE Class 10 Science Worksheet
Students can use the Chapter 04 Carbon and Its Compounds practice sheet provided above to prepare for their upcoming school tests. This solved questions and answers follow the latest CBSE syllabus for Class 10 Science. You can easily download the PDF format and solve these questions every day to improve your marks. Our expert teachers have made these from the most important topics that are always asked in your exams to help you get more marks in exams.
NCERT Based Questions and Solutions for Chapter 04 Carbon and Its Compounds
Our expert team has used the official NCERT book for Class 10 Science to create this practice material for students. After solving the questions our teachers have also suggested to study the NCERT solutions which will help you to understand the best way to solve problems in Science. You can get all this study material for free on studiestoday.com.
Extra Practice for Science
To get the best results in Class 10, students should try the Science MCQ Test for this chapter. We have also provided printable assignments for Class 10 Science on our website. Regular practice will help you feel more confident and get higher marks in CBSE examinations.
You can download the CBSE Practice worksheets for Class 10 Science Chapter 04 Carbon and Its Compounds for the latest session from StudiesToday.com
Yes, the Practice worksheets issued for Chapter 04 Carbon and Its Compounds Class 10 Science have been made available here for the latest academic session
There is no charge for the Practice worksheets for Class 10 CBSE Science Chapter 04 Carbon and Its Compounds you can download everything free
Regular revision of practice worksheets given on studiestoday for Class 10 subject Science Chapter 04 Carbon and Its Compounds can help you to score better marks in exams
Yes, studiestoday.com provides all the latest Class 10 Science Chapter 04 Carbon and Its Compounds test practice sheets with answers based on the latest books for the current academic session

