Practice NEET Chemistry The D and F Block Elements MCQs Set A provided below. The MCQ Questions for Full Syllabus The D and F Block Elements Chemistry with answers and follow the latest NEET/ NCERT and KVS patterns. Refer to more Chapter-wise MCQs for NEET Full Syllabus Chemistry and also download more latest study material for all subjects
MCQ for Full Syllabus Chemistry The D and F Block Elements
Full Syllabus Chemistry students should review the 50 questions and answers to strengthen understanding of core concepts in The D and F Block Elements
The D and F Block Elements MCQ Questions Full Syllabus Chemistry with Answers
Question: Which of the following does not have abnormal electronic configuration?
- a) Cr
- b) Pd
- c) Pt
- d) Hg
Answer: Hg
Question: The trend in ionisation enthalpy of a transition element is not regular because
- a) Removal of one electron alters the relative energies of 4s and 3d orbitals
- b) Due to different E.C. (stability)
- c) Poor screening of 3p orbital
- d) Both (1) & (2)
Answer: Removal of one electron alters the relative energies of 4s and 3d orbitals
Question: The element having lowest IE1
- a) Fe
- b) Co
- c) Ni
- d) Cu
Answer: Ni
Question: Choose the correct pair regarding IE3.
- a) Mn > Cr
- b) Mn > Fe
- c) Zn > Cu
- d) All of these
Answer: All of these
Question: Which of the following element does not show the variable oxidation state?
- a) Fe
- b) Mn
- c) Cu
- d) Zn
Answer: Zn
Question: With F highest stable oxidation state of Mn is
- a) +6
- b) +4
- c) +7
- d) +3
Answer: +4
Question: With O highest possible oxidation state of Mn is
- a) +7
- b) +4
- c) +5
- d) +3
Answer: +7
Question: Oxygen stabilises higher oxidation state because
- a) It is electronegative
- b) Of its tendency to form double bond
- c) Of small size
- d) Of large size
Answer: Of its tendency to form double bond
Question: Which of the following have highest magnetic moment?
- a) Fe2+
- b) Mn+
- c) Fe3+
- d) Fe+
Answer: Mn+
Question: Reduction potential of M2+/M will depend on
- a) IE1+ IE2
- b) ΔH atomisation
- c) Hydration energy
- d) All of these
Answer: All of these
Question: Amongst the following ions, which is considered as most stable in M2+ state?
- a) Ti2+/Ti (–1.63 V)
- b) V2+/V (–1.11 V)
- c) Cr2+/Cr (–0.90 V)
- d) Mn2+/Mn (1.18 V)
Answer: Ti2+/Ti (–1.63 V)
Question: Electrode potential of M2+/M for Ni is abnormal because of
- a) High IE1+ IE2
- b) High hydration energy
- c) ΔH atomisation
- d) Electronic configuration of Ni2+
Answer: High hydration energy
Question: Size of lanthanide decrease because of poor screening of
- a) 4f
- b) 3d
- c) 5f
- d) 4d
Answer: 4f
Question: The inner transition element that is radioactive is
- a) Pm
- b) Gd
- c) Lu
- d) Sm
Answer: Pm
More Questions........................................................
Question: The strongest base is
- a) Ce(OH)3
- b) Lu(OH)3
- c) Yb(OH)3
- d) Pm(OH)3
Answer: Ce(OH)3
Question: The lanthanide contraction is responsible for the fact that
- a) Zr and Y have about the same radius
- b) Zr and Nb have similar oxidation state
- c) Zr and Hf have about the same radius
- d) Zr and Zn have the same oxidation state
Answer: Zr and Hf have about the same radius
Question: The element that is not present in misch metal is
- a) La
- b) Iron
- c) Na
- d) Ce
Answer: Na
Question: Most stable oxidation state of Lanthanides
- a) +2
- b) +3
- c) +4
- d) +1
Answer: +3
Question: Which one of the following pairs of ions have same electronic configuration?
- a) Cr3+ Fe3+
- b) Mn2+, Fe3+
- c) Fe3+ Co3+
- d) Sc3+, Cr3+
Answer: Mn2+, Fe3+
Question: The species which is paramagnetic
- a) Cr+
- b) Zn2+
- c) Cu+
- d) MnO4–
Answer: Cr+
Question: When intimate mixture of potassium dichromate and potassium chloride is heated with conc. H2SO4 which of the following is produced in the form of red vapours?
- a) CrO3
- b) Cr2O3
- c) CrO2Cl2
- d) CrCl2
Answer: CrO2Cl2
Question: Which of the following oxide is basic?
- a) CrO
- b) Cr2O3
- c) CrO3
- d) Cr2O4
Answer: CrO
Question:
oxidised product of X. X in the above reaction cannot be
- a)
- b) Fe2+
- c)
- d) S2–
Answer:
Question: The reducing nature of any metal in aqueous solution depends upon a. Enthalpy of atomisation b. Ionisation enthalpies c. Hydration energy
- a) a & b only
- b) Only b
- c) b & c only
- d) a, b & c
Answer: a, b & c
Question: The magnetic moment of a transition metal ion is found to be 5.92 BM. The number of unpaired electrons present in it is
- a) 2
- b) 3
- c) 4
- d) 5
Answer: 5
Question: Which of the following is the consequences of lanthanide contraction?
- a) Separation of mixture of lanthanides is difficult
- b) Basic nature of hydroxides decrease from first member to last member of lanthanides
- c) Size of Hf and Zr is different
- d) Both (1) & (2)
Answer: Both (1) & (2)
Question: Which of the following is non-typical transition element?
- a) Ti
- b) Cr
- c) Fe
- d) Sc
Answer: Sc
Question: n-Factor of KMnO4 in neutral medium is
- a) 6
- b) 5
- c) 4
- d) 3
Answer: 3
Question: K2Cr2O7+ I– + H+ —→ Oxidized product. The product is
- a) KIO3
- b) I2
- c) I3–
- d) Cr2O3
Answer: I2
Question: Which of the following is coloured due to charge transfer?
- a) MnO4–
- b) CrO42–
- c) Cu2O
- d) All of these
Answer: All of these
Question: Coinage metals are
- a) Normal metals
- b) Transition metals
- c) Active metals
- d) Highly electropositive
Answer: Transition metals
Question: Pyrolusite is used to prepare potassium permanganate 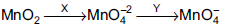 X and Y are
X and Y are
- a) Fuse with KOH/air, electrolytic reduction
- b) Fuse with KOH/air, electrolytic oxidation
- c) Fuse with con. HNO3 /air, electrolytic reduction
- d) All are correct
Answer: Fuse with KOH/air, electrolytic oxidation
Question: Which one of the following exhibits highest oxidation state?
- a) Zr
- b) V
- c) Mn
- d) Ni
Answer: Mn
Question: Ce(Z = 58) and Yb(Z = 70) exhibits stable +4 and +2 oxidation states respectively. This is because
- a) Ce4+ and Yb2+ acquire f7 configuration
- b) Ce4+ and Yb2+ acquire f0 configuration
- c) Ce4+ and Yb2+ acquire f0 and f14 configuration
- d) Ce4+ and Yb2+ acquire f7 and f14 configuration
Answer: Ce4+ and Yb2+ acquire f0 and f14 configuration
Question: A purple coloured solution is made alkaline with KOH and is treated with KI forming potassium iodate. The same solution is acidified with H2SO4 and again it H is treated with KI. However this time instead of potassium iodate, iodine gas is released. The purple coloured solution is of
- a) K2Cr2O7
- b) K2Cr2O4
- c) KMnO4
- d) K2MnO4
Answer: KMnO4
Question: FeSO4 on heating gives
- a) SO2 and SO3
- b) SO2 only
- c) SO3 only
- d) SO2 and O2
Answer: SO2 and SO3
Question: Acidified solution of chromic acid on treatment with H2O2 gives blue colour which is due to
- a) CrO3+ H2O + O2
- b) Cr2O3+ H2O + O2
- c) CrO5+ H2O
- d) H2Cr2O7+ H2O + CO2
Answer: CrO5+ H2O
Question: What are the species X and Y in the following?
- a)
- b) CrO3, Cr2O3
- c) H2CrO4, H2Cr2O7
- d)
Answer:
Question: The correct statement
- a) Green vitriol and blue vitriol are isomorphus
- b) KMnO4 and K2Cr2O7 are coloured due to d-d transitions
- c) Cu2Cl2 and Ag2S are coloured
- d) Upon strong heating paramagnetic gases are evolved by NaNO3 and AgNO3
Answer: Upon strong heating paramagnetic gases are evolved by NaNO3 and AgNO3
Question: Which oxide of manganese is acidic in nature?
- a) MnO
- b) Mn2O7
- c) Mn2O3
- d) MnO2
Answer: Mn2O7
Question: The blue colour produced on adding H2O2 to acidified K2Cr2O7 is due to the formation of
- a) CrO5
- b) Cr2O3
- c) CrO42—
- d) CrO3
Answer: CrO5
Question: Ammonium dichromate is used in fireworks.The green coloured powder blown in the air is
- a) CrO3
- b) Cr2O3
- c) Cr
- d) CrO(O)2
Answer: Cr2O3
Question: Which of the following statement is correct for 3d-transition element?
- a) All the metals except Sc forms 'MO' oxide
- b) All the metals except Zn forms 'MO' oxide
- c) All the metals except Zn and Sc form 'MO' oxide
- d) All the metals except Mn forms 'MO' oxide
Answer: All the metals except Sc forms 'MO' oxide
Question: 4K2Cr2O7—→ 4K2CrO4+ 3O2+ X, in this reaction X is
- a) CrO3
- b) Cr2O7
- c) Cr2O3
- d) CrO5
Answer: Cr2O3
Question: Which of the following is not coloured?
- a) Mn2+
- b) Cr3+
- c) Zn2+
- d) Cu2+
Answer: Zn2+
Question: Which of the following belongs to group ‘8’?
- a) Ni, Pd, Pt
- b) F, Cl, Br
- c) Fe, Ru, Os
- d) Xe, Ar, Kr
Answer: Fe, Ru, Os
Question: The equivalent weight of MnSO4 is equal to its molecular weight when it is converted to
- a) Mn2O3
- b) MnO2
- c) MnO4–
- d) MnO42–
Answer: Mn2O3
Question: Which one of the following pairs of ions have same electronic configuration?
- a) Cr3+ Fe3+
- b) Mn2+, Fe3+
- c) Fe3+ Co3+
- d) Sc3+, Cr3+
Answer: Mn2+, Fe3+
Question: Transuranic elements begin with
- a) Np
- b) Cm
- c) Pu
- d) U
Answer: Np
Question: The element which does not show d0 configuration in its highest oxidation state
- a) V
- b) Mn
- c) Cr
- d) Fe
Answer: Fe
Question: Gun metal contains
- a) Cu, Sn, Zn
- b) Cu, Ni
- c) Cu, Ni, Fe
- d) Cu, Sn, P
Answer: Cu, Sn, Zn
Question: The colour of K2Cr2O7 and Fe2+ ions are respectively due to
- a) d-d transition and charge transfer spectra
- b) Charge transfer spectra and d-d transition
- c) Crystal defects and charge transfer spectra
- d) Charge transfer spectra and crystal defects
Answer: Charge transfer spectra and d-d transition
Question: CrO3 is coloured due to
- a) Crystal defect
- b) Unpaired electrons
- c) Charge transfer spectra
- d) Low I.E.
Answer: Charge transfer spectra
Question: Which of the following occur when AgNO3 becomes, red hot?
- a) 2AgNO3—→ 2Ag + 2NO2+ O2
- b) AgNO3—→ Ag + NO + O2
- c) 2AgNO3—→ AgNO2+ O2
- d) 2AgNO3—→ 2Ag + N2+ 3O2
Answer: 2AgNO3—→ 2Ag + 2NO2+ O2
Question: Which one alloy does not contain copper?
- a) Bronze
- b) Brass
- c) German silver
- d) Mischmetal
Answer: Mischmetal
Question: The metal which can form cation having metal - metal bond
- a) Mercury
- b) Copper
- c) Osmium
- d) Iron
Answer: Mercury
Question: Value of magnetic moment of a divalent metal ion is 5.92 BM. Total number of electron in its atom would be
- a) 24
- b) 25
- c) 26
- d) 27
Answer: 25
Question: In black and white photography, the developed film is fixed by washing with
- a) AgBr solution
- b) Hypo solution
- c) Na2S4O6 solution
- d) FeC2O4 solution
Answer: Hypo solution
Question: Gold dissolves in aqua regia to give
- a) H[AuCl4]
- b) AuNO3
- c) H2[AuCl6]
- d) Au(NO3)3
Answer: H[AuCl4]
Question: Which of the following belongs to group ‘8’?
- a) Ni, Pd, Pt
- b) F, Cl, Br
- c) Fe, Ru, Os
- d) Xe, Ar, Kr
Answer: Fe, Ru, Os
| NEET Chemistry Alcohols Phenols and Ethers MCQs Set A |
| NEET Chemistry Alcohols Phenols and Ethers MCQs Set B |
| NEET Chemistry Aldehydes Ketones and Carboxylic Acids MCQs Set A |
| NEET Chemistry Aldehydes Ketones and Carboxylic Acids MCQs Set B |
| NEET UG Chemistry Biomolecule MCQs |
| NEET UG Chemistry Chemical Bonding MCQs |
| NEET Chemistry Chemical Bonding and Molecular Structure MCQs Set A |
| NEET Chemistry Chemical Bonding and Molecular Structure MCQs Set B |
| NEET Chemistry Chemical Kinetics MCQs Set A |
| NEET Chemistry Chemical Kinetics MCQs Set B |
| NEET UG Chemistry Chemical Kinetics MCQs |
| NEET UG Chemistry Chemical Thermodynamics MCQs |
| NEET Chemistry Chemistry In Everyday Life MCQs Set A |
| NEET Chemistry Chemistry In Everyday Life MCQs Set B |
| NEET UG Chemistry in Everyday Life MCQs |
| NEET UG Chemistry Classification of Elements MCQs |
| NEET Chemistry Classification Of Elements and Periodicity In Properties MCQs Set A |
| NEET Chemistry Classification Of Elements and Periodicity In Properties MCQs Set B |
| NEET UG Chemistry D and F Block Elements MCQs |
| NEET Chemistry Electrochemistry MCQs Set A |
| NEET Chemistry Electrochemistry MCQs Set B |
| NEET Chemistry Electrochemistry MCQs Set C |
| NEET Chemistry Environmental Chemistry MCQs Set A |
| NEET Chemistry Environmental Chemistry MCQs Set B |
| NEET UG Chemistry Environmental Chemistry MCQs |
| NEET Chemistry Equilibrium MCQs Set A |
| NEET Chemistry Equilibrium MCQs Set B |
| NEET Chemistry Equilibrium MCQs Set C |
| NEET UG Chemistry Equilibrium MCQs |
| NEET Chemistry General Principles and Processes Of Isolation Of Elements MCQs Set A |
| NEET Chemistry General Principles and Processes Of Isolation Of Elements MCQs Set B |
| NEET Chemistry Haloalkanes and Haloarenes MCQs Set A |
| NEET Chemistry Haloalkanes and Haloarenes MCQs Set B |
| NEET Chemistry Hydrocarbons MCQs Set A |
| NEET Chemistry Hydrocarbons MCQs Set B |
| NEET Chemistry Hydrocarbons MCQs Set C |
| NEET UG Chemistry Hydrocarbons MCQs |
| NEET Chemistry Hydrogen MCQs Set A |
| NEET Chemistry Hydrogen MCQs Set B |
| NEET UG Chemistry Hydrogen MCQs |
| NEET UG Chemistry Isolation of Metals MCQs |
| NEET UG Chemistry Organic Chemistry MCQs |
| NEET UG Chemistry Organic Compounds Containing Halogens MCQs |
| NEET UG Chemistry Organic Compound Containing Nitrogen MCQs |
| NEET UG Chemistry Organic Compounds MCQs |
| NEET UG Chemistry Organic Compounds Containing Oxygen MCQs |
| NEET UG Chemistry P Block Elements MCQs |
| NEET UG Chemistry Practicals MCQs |
| NEET UG Chemistry Redox Reactions and Electrochemistry MCQs |
| NEET UG Chemistry S Block Elements MCQs |
| NEET Chemistry Solutions MCQs Set A |
| NEET Chemistry Solutions MCQs Set B |
| NEET UG Chemistry Solutions MCQs |
| NEET Chemistry Some Basic Concepts Of Chemistry MCQs Set A |
| NEET Chemistry Some Basic Concepts Of Chemistry MCQs Set B |
| NEET UG Chemistry Some Basic Concepts MCQs |
| NEET Chemistry States Of Matter MCQs Set A |
| NEET Chemistry States Of Matter MCQs Set B |
| NEET UG Chemistry States of Matter MCQs |
| NEET Chemistry Structure Of Atom MCQs Set A |
| NEET Chemistry Structure Of Atom MCQs Set B |
| NEET UG Chemistry Structure of Atom MCQs |
| NEET Chemistry Surface Chemistry MCQs Set A |
| NEET UG Chemistry Surface Chemistry MCQs |
| NEET Chemistry The D and F Block Elements MCQs Set A |
| NEET Chemistry The D and F Block Elements MCQs Set B |
| NEET Chemistry The P Block Elements MCQs Set A |
| NEET Chemistry The P Block Elements MCQs Set B |
| NEET Chemistry The P Block Elements MCQs Set C |
Important Practice Resources for NEET Chemistry Advanced Study Material
MCQs for The D and F Block Elements Chemistry Full Syllabus
Students can use these MCQs for The D and F Block Elements to quickly test their knowledge of the chapter. These multiple-choice questions have been designed as per the latest syllabus for Full Syllabus Chemistry released by NEET. Our expert teachers suggest that you should practice daily and solving these objective questions of The D and F Block Elements to understand the important concepts and better marks in your school tests.
The D and F Block Elements NCERT Based Objective Questions
Our expert teachers have designed these Chemistry MCQs based on the official NCERT book for Full Syllabus. We have identified all questions from the most important topics that are always asked in exams. After solving these, please compare your choices with our provided answers. For better understanding of The D and F Block Elements, you should also refer to our NCERT solutions for Full Syllabus Chemistry created by our team.
Online Practice and Revision for The D and F Block Elements Chemistry
To prepare for your exams you should also take the Full Syllabus Chemistry MCQ Test for this chapter on our website. This will help you improve your speed and accuracy and its also free for you. Regular revision of these Chemistry topics will make you an expert in all important chapters of your course.
You can get most exhaustive NEET Chemistry The D and F Block Elements MCQs Set A for free on StudiesToday.com. These MCQs for Full Syllabus Chemistry are updated for the 2025-26 academic session as per NEET examination standards.
Yes, our NEET Chemistry The D and F Block Elements MCQs Set A include the latest type of questions, such as Assertion-Reasoning and Case-based MCQs. 50% of the NEET paper is now competency-based.
By solving our NEET Chemistry The D and F Block Elements MCQs Set A, Full Syllabus students can improve their accuracy and speed which is important as objective questions provide a chance to secure 100% marks in the Chemistry.
Yes, Chemistry MCQs for Full Syllabus have answer key and brief explanations to help students understand logic behind the correct option as its important for 2026 competency-focused NEET exams.
Yes, you can also access online interactive tests for NEET Chemistry The D and F Block Elements MCQs Set A on StudiesToday.com as they provide instant answers and score to help you track your progress in Chemistry.

