Practice NEET Chemistry Some Basic Concepts Of Chemistry MCQs Set A provided below. The MCQ Questions for Full Syllabus Some Basic Concepts Of Chemistry Chemistry with answers and follow the latest NEET/ NCERT and KVS patterns. Refer to more Chapter-wise MCQs for NEET Full Syllabus Chemistry and also download more latest study material for all subjects
MCQ for Full Syllabus Chemistry Some Basic Concepts Of Chemistry
Full Syllabus Chemistry students should review the 50 questions and answers to strengthen understanding of core concepts in Some Basic Concepts Of Chemistry
Some Basic Concepts Of Chemistry MCQ Questions Full Syllabus Chemistry with Answers
Question: A sample of ammonium phosphate (NH 4)3 PO 4 contains 3.18 moles of hydrogen atoms. The number of moles of oxygen atoms in the sample is
- a) 0.265
- b) 0.795
- c) 1.06
- d) 4.00
Answer: 1.06
Question: In which of the following numbers all zeros are significant?
- a) 0.0007
- b) 0.0070
- c) 70.000
- d) 0.700
Answer: 70.000
Question: Two metallic oxides contain 27.6% and 30% oxygen respectively. If the formula of the first oxide is X3O4 , that of the second will be
- a) XO
- b) XO2
- c) X2O5
- d) X2O3
Answer: X2O3
Question: Calculate the molality of solution containing 3 g glucose dissolved in 30 g of water. (molar mass of glucose = 180)
- a) 0.50 m
- b) 0.56 m
- c) 0.091 m
- d) 0.05 m
Answer: 0.56 m
Question: An element, X has the following isotopic composition 200X : 90% 199X : 8% 202X : 2.0%. The weighted average atomic mass of the naturally occurring element X is closest to
- a) 201 amu
- b) 202 amu
- c) 199 amu
- d) 200 amu
Answer: 200 amu
Question: Which has the maximum number of molecules among the following?
- a) 8 g H2
- b) 64 g SO2
- c) 44 g CO2
- d) 48 g O3
Answer: 8 g H2
Question: What volume of oxygen gas (O2) measured at 0°C and 1 atm, is needed to burn completely 1 L of propane gas (C3H8) measured under the same conditions?
- a) 10 L
- b) 7 L
- c) 6 L
- d) 5 L
Answer: 5 L
Question: Volume occupied by one molecule of water (density = 1 g cm–3) is
- a) 5.5 × 10–23 cm3
- b) 9.0 × 10–23 cm3
- c) 6.023 × 10–23 cm3
- d) 3.0 × 10–23 cm3
Answer: 3.0 × 10–23 cm3
Question: The total number of electrons in 1.6 g of CH4 to that in 1.8 g of H2O
- a) Double
- b) Same
- c) Triple
- d) One fourth
Answer: Same
Question: When x molecules are removed from 200 mg of N2O, 2.89 × 10–3 moles of N2O are left. x will be
- a) 1020 molecules
- b) 1010 molecules
- c) 21 molecules
- d) 1021 molecules
Answer: 1021 molecules
Question: 4 g of hydrogen reacts with 20 g of oxygen to form water. The mass of water formed is
- a) 24 g
- b) 36 g
- c) 22.5 g
- d) 40 g
Answer: 22.5 g
Question: Which has maximum molecules?
- a) 7 g N2O
- b) 20 g H2
- c) 16 g NO2
- d) 16 g SO2
Answer: 20 g H2
Question: The number of molecules in 4.25 g of NH3 is approximately
- a) 4 × 1023
- b) 1.5 × 2023
- c) 1 × 1023
- d) 6 × 1023
Answer: 1.5 × 2023
Question: The maximum number of molecules is present in
- a) 15 L of H2 gas at STP
- b) 5 L of N2 gas at STP
- c) 0.5 g of H2 gas
- d) 10 g of O2 gas
Answer: 15 L of H2 gas at STP
Question: The number of atoms in 0.1 mol of a tetraatomic gas is (NA= 6.02 × 1023 mol–1)
- a) 2.4 × 1022
- b) 6.026 × 1022
- c) 2.4 × 1023
- d) 3.600 × 1023
Answer: 2.4 × 1023
Question: How many grams of NaOH should be added to water to prepare 250 ml solution of 2 M NaOH?
- a) 9.6 × 103
- b) 2.4 × 103
- c) 20
- d) 24
Answer: 20
Question: Haemoglobin contains 0.334% of iron by weight. The molecular weight of haemoglobin is approximately 67200. The number of iron atoms (Atomic weight of Fe is 56) present in one molecule of haemoglobin is
- a) 4
- b) 6
- c) 3
- d) 2
Answer: 4
Question: In the reaction,
when 1 mole of SO2 and 1 mole of O2 are made to react to completion
- a) All the oxygen will be consumed
- b) 1.0 mole of SO3 will be produced
- c) 0.5 mole of SO2 is remained
- d) All of these
Answer: 1.0 mole of SO3 will be produced
Question: If the weight of metal chloride is x gram containing y gram of metal, the equivalent weight of metal will be
- a)
- b)
- c)
- d)
Answer:
Question: 10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be
- a) 1 mol
- b) 2 mol
- c) 3 mol
- d) 4 mol
Answer: 4 mol
More Questions........................................................
Question: The total number of electrons in 4.2 g of N3– ion is (NA is the Avogadro’s number)
- a) 2.1 NA
- b) 4.2 NA
- c) 3 NA
- d) 3.2 NA
Answer: 3 NA
Question: The number of mole of nitrogen in one litre of air containing 10% nitrogen by volume, under standard conditions, is
- a) 0.03 mole
- b) 2.10 moles
- c) 0.186 mole
- d) 4.46 × 10–3 mole
Answer: 4.46 × 10–3 mole
Question: Liquid benzene (C6H6 ) burns in oxygen according to
How many litres of O2 at STP are needed to complete the combustion of 39 g of liquid benzene?
- a) 74 L
- b) 11.2 L
- c) 22.4 L
- d) 84 L
Answer: 84 L
Question: 1 mol of KClO3 is thermally decomposed and excess of aluminium is burnt in the gaseous product. How many moles of Al2O3 are formed?
- a) 1
- b) 2
- c) 1.5
- d) 3
Answer: 1
Question: The amount of zinc required to produce 1.12 ml of H2 at STP on treatment with dilute HCl will be
- a) 65 g
- b) 0.065 g
- c) 32.5 × 10–4 g
- d) 6.5 g
Answer: 32.5 × 10–4 g
Question: Volume of CO2 obtained at STP by the complete decomposition of 9.85 g Na2 CO3 is
- a) 2.24 litre
- b) Zero litre
- c) 0.85 litre
- d) 0.56 litre
Answer: Zero litre
Question: One litre of CO2 is passed through red hot coke. The volume becomes 1.4 litres at same temperature and pressure. The composition of products is
- a) 0.8 litre of CO2 and 0.6 litre of CO
- b) 0.7 litre of CO2 and 0.7 litre of CO
- c) 0.6 litre of CO2 and 0.8 litre of CO
- d) 0.4 litre of CO2 and 1.0 litre of CO
Answer: 0.6 litre of CO2 and 0.8 litre of CO
Question: Suppose that A and B form the compounds B2A3 and B2A. If 0.05 mole of B2A3 weighs 9 g and 0.1 mole of B2 A weighs 10 g, the atomic weight of A and B respectively are
- a) 30 and 40
- b) 40 and 30
- c) 20 and 5
- d) 15 and 20
Answer: 40 and 30
Question: When 100 ml of
is mixed with 500 ml of
NaOH then nature of resulting solution and normality of excess of reactant left is
- a)
- b)
- c)
- d)
Answer:
Question: Mole fraction of solvent in aqueous solution of NaOH having molality of 3 is
- a) 0.3
- b) 0.05
- c) 0.7
- d) 0.95
Answer: 0.95
Question: Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 gmL–1. Volume of acid required to make one litre of 0.1 M H2SO4 solution is
- a) 16.65 mL
- b) 22.20 mL
- c) 5.55 mL
- d) 11.10 mL
Answer: 5.55 mL
Question: Number of significant figures in 6.62 × 10–34.
- a) Two
- b) Three
- c) Four
- d) One
Answer: Three
Question: Ammonia gas is passed into water, yielding a solution of density 0.93 g/cm3 and containing 18.6% NH3 by weight. The mass of NH3 per cc of the solution is
- a) 0.17 g/cm3
- b) 0.34 g/cm3
- c) 0.51 g/cm3
- d) 0.68 g/cm3
Answer: 0.17 g/cm3
Question: A certain amount of a metal whose equivalent mass is 28 displaces 0.7 L of H2 at S.T.P. from an acid hence mass of the element is
- a) 1.75 g
- b) 0.875 g
- c) 3.50 g
- d) 7.00 g
Answer: 1.75 g
Question: Number of Fe atoms in 100 g Haemoglobin if it contains 0.33% Fe. (Atomic mass of Fe = 56)
- a) 0.035 × 1023
- b) 35
- c) 3.5 × 1023
- d) 7 × 108
Answer: 0.035 × 1023
Question: An organic compound containing C and H gave the following analysis C = 40%, H = 6.7%. Its empirical formula would be
- a) CH4
- b) CH2O
- c) C2H4O2
- d) C2H4
Answer: CH2O
Question: The number of electrons in 1.6 g of CH4 is approximately
- a) 25 × 1024
- b) 1.5 × 1024
- c) 6 × 1023
- d) 3.0 × 1024
Answer: 6 × 1023
Question: 6.025 × 1020 molecules of acetic acid are present in 500 ml of its solution. The concentration of solution is
- a) 0.002 M
- b) 10.2 M
- c) 0.012 M
- d) 0.001 M
Answer: 0.002 M
Question: How many litre of oxygen at STP is required to burn 60 g C2H6 ?
- a) 22.4 L
- b) 11.2 L
- c) 22.4 × 7 L
- d) 8.5 L
Answer: 22.4 × 7 L
Question: For the formation of 3.65 g of HCl gas, what volume of hydrogen gas and chlorine gas are required at NTP conditions?
- a) 1 L, 1 L
- b) 1.12 L, 2.24 L
- c) 3.65 L, 1.83 L
- d) 1.12 L, 1.12 L
Answer: 1.12 L, 1.12 L
Question: A mixture of gases contains H2and O2 gases in the ratio of 1 : 4 (w/w). What is the molar ratio of the two gases in the mixture?
- a) 2 : 1
- b) 1 : 4
- c) 4 : 1
- d) 16 : 1
Answer: 4 : 1
Question: Specific volume of cylindrical virus particle is 6.02 × 10–2 cc/gm whose radius and length are 7 Å and 10 Å respectively. If NA= 6.02 × 1023, find molecular weight of virus.
- a) 15.4 kg/mol
- b) 1.54 × 104 kg/mol
- c) 3.08 × 104 kg/mol
- d) 3.08 × 103 kg/mol
Answer: 15.4 kg/mol
Question: 1.0 g of magnesium is burnt with 0.56 g O2 in a closed vessel. Which reactant is left in excess and how much? (At. wt. Mg = 24; O = 16)
- a) Mg, 0.16 g
- b) O2, 0.16 g
- c) Mg, 0.44 g
- d) O2, 0.28 g
Answer: Mg, 0.16 g
Question: When 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2 (g), each at STP, the moles of HCl(g) formed is equal to
- a) 1 mol of HCl(g)
- b) 2 mol of HCl(g)
- c) 0.5 mol of HCl(g)
- d) 1.5 mol of HCl(g)
Answer: 1 mol of HCl(g)
Question: 6.02 × 1020 molecules of urea are present in 100 mL of its solution. The concentration of solution is
- a) 0.01 M
- b) 0.001 M
- c) 0.1 M
- d) 0.02 M
Answer: 0.01 M
Question: How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2 M HNO3 ? The concentrated acid is 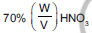 HNO3
HNO3
- a) 90.0 g conc. HNO3
- b) 70.0 g conc. HNO3
- c) 54.0 g conc. HNO3
- d) 45.0 g conc. HNO3
Answer: 45.0 g conc. HNO3
Question: Mole fraction of the solute in a 1.00 molal aqueous solution is
- a) 1.7700
- b) 0.1770
- c) 0.0177
- d) 0.0344
Answer: 0.0177
Question: Which has the maximum number of molecules among the following?
- a) 8 g H2
- b) 64 g SO2
- c) 44 g CO2
- d) 48 g O3
Answer: 8 g H2
Question: The number of atoms in 0.1 mol of a triatomic gas is (NA =6.02 × 1023 mol–1)
- a) 6.026 × 1022
- b) 1.806 × 1023
- c) 3.600 × 1023
- d) 1.800 × 1022
Answer: 1.806 × 1023
Question: 25.3 g of sodium carbonate, Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ion, Na+ and carbonate ions,
are respectively (Molar mass of Na2CO3= 106 g mol–1)
- a) 0.955 M and 1.910 M
- b) 1.910 M and 0.955 M
- c) 1.90 M and 1.910 M
- d) 0.477 M and 0.477 M
Answer: 1.910 M and 0.955 M
Question: 10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be
- a) 3 mol
- b) 4 mol
- c) 1 mol
- d) 2 mol
Answer: 4 mol
Question: How many moles of lead (II) chloride will be formed from a reaction between 6.5g of PbO and 3.2 g of HCl?
- a) 0.029
- b) 0.044
- c) 0.333
- d) 0.011
Answer: 0.029
Question: Volume occupied by one molecule of water (density = 1 g cm–3) is
- a) 5.5 × 10–23 cm3
- b) 9.0 × 10–23 cm3
- c) 6.023 × 10–23 cm3
- d) 3.0 × 10–23 cm3
Answer: 3.0 × 10–23 cm3
Question: What volume of oxygen gas (O2) measured at 0°C and 1 atm, is needed to burn completely 1 L of propane gas (C3H8) measured under the same conditions?
- a) 10 L
- b) 7 L
- c) 6 L
- d) 5 L
Answer: 5 L
Question: An element, X has the following isotopic composition ; 200X : 90% ; 199X : 8.0% ; 202X : 2.0% The weighted average atomic mass of the naturally occurring element X is closest to :
- a) 199 amu
- b) 200 amu
- c) 201 amu
- d) 202 amu
Answer: 200 amu
Question: Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 g mL–1. Volume of acid required to make one litre of 0.1 M H2SO4 is
- a) 5.55 mL
- b) 11.10 mL
- c) 16.65 mL
- d) 22.20 mL
Answer: 5.55 mL
Question: An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave C, 38.71% and H, 9.67%. The empirical formula of the compound would be
- a) CH4O
- b) CH3O
- c) CH2O
- d) CHO
Answer: CH3O
Question: How many grams of CH3OH should be added to water to prepare 150 ml solution of 2 M CH3OH?
- a) 9.6 × 103
- b) 2.4 × 103
- c) 9.6
- d) 2.4
Answer: 9.6
Question: The number of mole of oxygen in one litre of air containing 21% oxygen by volume, under standard conditions, is
- a) 0.0093 mole
- b) 2.10 moles
- c) 0.186 mole
- d) 0.21 mole
Answer: 0.0093 mole
Question: The total number of valence electrons in 4.2 g of N3– ion is (NA is the Avogadro’s number)
- a) 2.1 NA
- b) 4.2 NA
- c) 1.6 NA
- d) 3.2 NA
Answer: 1.6 NA
| NEET Chemistry Alcohols Phenols and Ethers MCQs Set A |
| NEET Chemistry Alcohols Phenols and Ethers MCQs Set B |
| NEET Chemistry Aldehydes Ketones and Carboxylic Acids MCQs Set A |
| NEET Chemistry Aldehydes Ketones and Carboxylic Acids MCQs Set B |
| NEET UG Chemistry Biomolecule MCQs |
| NEET UG Chemistry Chemical Bonding MCQs |
| NEET Chemistry Chemical Bonding and Molecular Structure MCQs Set A |
| NEET Chemistry Chemical Bonding and Molecular Structure MCQs Set B |
| NEET Chemistry Chemical Kinetics MCQs Set A |
| NEET Chemistry Chemical Kinetics MCQs Set B |
| NEET UG Chemistry Chemical Kinetics MCQs |
| NEET UG Chemistry Chemical Thermodynamics MCQs |
| NEET Chemistry Chemistry In Everyday Life MCQs Set A |
| NEET Chemistry Chemistry In Everyday Life MCQs Set B |
| NEET UG Chemistry in Everyday Life MCQs |
| NEET Chemistry States Of Matter MCQs Set A |
| NEET Chemistry States Of Matter MCQs Set B |
| NEET UG Chemistry Classification of Elements MCQs |
| NEET Chemistry Classification Of Elements and Periodicity In Properties MCQs Set A |
| NEET Chemistry Classification Of Elements and Periodicity In Properties MCQs Set B |
| NEET UG Chemistry D and F Block Elements MCQs |
| NEET Chemistry Electrochemistry MCQs Set A |
| NEET Chemistry Electrochemistry MCQs Set B |
| NEET Chemistry Electrochemistry MCQs Set C |
| NEET Chemistry Environmental Chemistry MCQs Set A |
| NEET Chemistry Environmental Chemistry MCQs Set B |
| NEET UG Chemistry Environmental Chemistry MCQs |
| NEET Chemistry Equilibrium MCQs Set A |
| NEET Chemistry Equilibrium MCQs Set B |
| NEET Chemistry Equilibrium MCQs Set C |
| NEET UG Chemistry Equilibrium MCQs |
| NEET Chemistry General Principles and Processes Of Isolation Of Elements MCQs Set A |
| NEET Chemistry General Principles and Processes Of Isolation Of Elements MCQs Set B |
| NEET Chemistry Haloalkanes and Haloarenes MCQs Set A |
| NEET Chemistry Haloalkanes and Haloarenes MCQs Set B |
| NEET Chemistry Hydrocarbons MCQs Set A |
| NEET Chemistry Hydrocarbons MCQs Set B |
| NEET Chemistry Hydrocarbons MCQs Set C |
| NEET UG Chemistry Hydrocarbons MCQs |
| NEET Chemistry Hydrogen MCQs Set A |
| NEET Chemistry Hydrogen MCQs Set B |
| NEET UG Chemistry Hydrogen MCQs |
| NEET UG Chemistry Isolation of Metals MCQs |
| NEET UG Chemistry Organic Chemistry MCQs |
| NEET UG Chemistry Organic Compounds Containing Halogens MCQs |
| NEET UG Chemistry Organic Compound Containing Nitrogen MCQs |
| NEET UG Chemistry Organic Compounds MCQs |
| NEET UG Chemistry Organic Compounds Containing Oxygen MCQs |
| NEET UG Chemistry P Block Elements MCQs |
| NEET UG Chemistry Practicals MCQs |
| NEET UG Chemistry Redox Reactions and Electrochemistry MCQs |
| NEET UG Chemistry S Block Elements MCQs |
| NEET Chemistry Solutions MCQs Set A |
| NEET Chemistry Solutions MCQs Set B |
| NEET UG Chemistry Solutions MCQs |
| NEET Chemistry Some Basic Concepts Of Chemistry MCQs Set A |
| NEET Chemistry Some Basic Concepts Of Chemistry MCQs Set B |
| NEET UG Chemistry Some Basic Concepts MCQs |
| NEET UG Chemistry States of Matter MCQs |
| NEET Chemistry Structure Of Atom MCQs Set A |
| NEET Chemistry Structure Of Atom MCQs Set B |
| NEET UG Chemistry Structure of Atom MCQs |
| NEET Chemistry Surface Chemistry MCQs Set A |
| NEET UG Chemistry Surface Chemistry MCQs |
| NEET Chemistry The D and F Block Elements MCQs Set A |
| NEET Chemistry The D and F Block Elements MCQs Set B |
| NEET Chemistry The P Block Elements MCQs Set A |
| NEET Chemistry The P Block Elements MCQs Set B |
| NEET Chemistry The P Block Elements MCQs Set C |
Important Practice Resources for NEET Chemistry Advanced Study Material
MCQs for Some Basic Concepts Of Chemistry Chemistry Full Syllabus
Expert teachers of studiestoday have referred to NCERT book for Full Syllabus Chemistry to develop the Chemistry Full Syllabus MCQs. If you download MCQs with answers for the above chapter you will get higher and better marks in Full Syllabus test and exams in the current year as you will be able to have stronger understanding of all concepts. Daily Multiple Choice Questions practice of Chemistry will help students to have stronger understanding of all concepts and also make them expert on all critical topics. After solving the questions given in the MCQs which have been developed as per latest books also refer to the NCERT solutions for Full Syllabus Chemistry. We have also provided lot of MCQ questions for Full Syllabus Chemistry so that you can solve questions relating to all topics given in each chapter. After solving these you should also refer to Full Syllabus Chemistry MCQ Test for the same chapter.
You can download the NEET MCQs for Full Syllabus Chemistry Some Basic Concepts Of Chemistry for latest session from StudiesToday.com
Yes, the MCQs issued by NEET for Full Syllabus Chemistry Some Basic Concepts Of Chemistry have been made available here for latest academic session
You can find NEET Full Syllabus Chemistry Some Basic Concepts Of Chemistry MCQs on educational websites like studiestoday.com, online tutoring platforms, and in sample question papers provided on this website.
To prepare for Some Basic Concepts Of Chemistry MCQs, refer to the concepts links provided by our teachers and download sample papers for free.
Yes, there are many online resources that we have provided on studiestoday.com available such as practice worksheets, question papers, and online tests for learning MCQs for Full Syllabus Chemistry Some Basic Concepts Of Chemistry

