Practice NEET Chemistry Hydrogen MCQs Set B provided below. The MCQ Questions for Full Syllabus Hydrogen Chemistry with answers and follow the latest NEET/ NCERT and KVS patterns. Refer to more Chapter-wise MCQs for NEET Full Syllabus Chemistry and also download more latest study material for all subjects
MCQ for Full Syllabus Chemistry Hydrogen
Full Syllabus Chemistry students should review the 50 questions and answers to strengthen understanding of core concepts in Hydrogen
Hydrogen MCQ Questions Full Syllabus Chemistry with Answers
Question: 20 volume hydrogen peroxide means
- a) 1 ml of H2O2 solution gives 20 L of O2 at NTP
- b) 1 mole of H2O2 give 20 ml of O2 at NTP
- c) 1 g of H2O2 give 20 ml of O2 at NTP
- d) 1 ml of H2O2 solution give 20 ml of O2 at NTP
Answer: 1 ml of H2O2 solution give 20 ml of O2 at NTP
Question: 1 ml of H2O2 solution gives 10 ml of O2 at N.T.P. It is
- a) 10 vol H2O2
- b) 20 vol H2O2
- c) 30 vol H2O2
- d) 40 H2O2
Answer: 10 vol H2O2
Question: The normality of 10 volume H2O2 is nearly
- a) 2.1
- b) 3.4
- c) 1.7
- d) 5.1
Answer: 1.7
Question: The amount of H2O2 present in 1 L of 1.5 N H2O2 solution is
- a) 2.5 g
- b) 25.5 g
- c) 3.0
- d) 8.0
Answer: 25.5 g
Question: H2O and H2O2 resemble in
- a) Hybridisation of oxygen
- b) Oxidation state of oxygen
- c) Structure
- d) Bond angle
Answer: Hybridisation of oxygen
Question: Boiling point of D2O is
- a) 100°C
- b) 105.5°C
- c) 101.4°C
- d) 102.6°C
Answer: 101.4°C
Question: In acidic medium, H2O2 changes 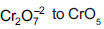 which has two (–O–O–) bonds. Oxidation state of Cr in CrO5 is
which has two (–O–O–) bonds. Oxidation state of Cr in CrO5 is
- a) +5
- b) +3
- c) +6
- d) –10
Answer: +6
Question: Some statements about heavy water are given below a. Heavy water is used as a moderator in nuclear reactors b. Heavy water is more associated than ordinary water c. Heavy water is more effective solvent than ordinary water Which of the above statements are correct ?
- a) a and b
- b) a, b and c
- c) b and c
- d) a and c
Answer: a and b
Question: Hydrogen is prepared from H2O by adding
- a) Ca, which acts as reducing agent
- b) Al, which acts as oxidising agent
- c) Ag, which acts as reducing agent
- d) Au, which acts as oxidising agent
Answer: Ca, which acts as reducing agent
Question: The hydride ion H– is stronger base than its hydroxide ion OH–. Which of the following reaction will occur if sodium hydride (NaH) is dissolved in water?
- a) H– + H2O → No reaction
- b) H– (aq) + H2O →H2O
- c) H– (aq) + H2O(l) →OH– + H2
- d) None of these
Answer: H– (aq) + H2O(l) →OH– + H2
Question: Which of the following statements about the interstitial compounds is incorrect?
- a) They are chemically reactive
- b) They are much harder than the pure metal
- c) They have higher melting points than the pure metal
- d) They retain metallic conductivity
Answer: They are chemically reactive
More Questions.......................................
Question: The volume strength of 1.5 NH2O2 solution is
- a) 8.8
- b) 8.4
- c) 4.8
- d) 5.2
Answer: 8.4
Question: Which one of the following pairs of substances on reaction will not evolve H2 gas?
- a) Copper and HCl (aqueous)
- b) Iron and steam
- c) Iron and H2SO4(aqueous)
- d) Sodium and ethyl alcohol
Answer: Copper and HCl (aqueous)
Question: The ease of adsorption of the hydrates alkali metal ions on an ion-exchange resins follows the order
- a) K+ < Na+< Rb+ < Li+
- b) Na+ < Li+ < K+ < Rb+
- c) Li+ < K+ < Na+ < Rb+
- d) Rb+ < K+ < Na+ < Li+
Answer: Li+ < K+ < Na+ < Rb+
Question: Zn gives H2 gas with H2SO4 and HCl but not with HNO3 because
- a) Zn act as oxidizing agent when react with HNO3
- b) HNO3 is weaker acid than H2SO4 and HCl
- c) In electrochemical series Zn is above hydrogen
- d)
is reduced in preference to hydronium ion
Answer: is reduced in preference to hydronium ion
Question: Which pair of substances gives same gaseous product, when these react with water?
- a) K and KO2
- b) Ba and BaO2
- c) Ca and CaH2
- d) Na and Na2O2
Answer: Ca and CaH2
Question: Among the following, identify the compound which cannot act as both oxidising and reducing agents
- a) H2O2
- b) H2
- c) SO2
- d) HNO2
Answer: H2
Question: Ortho and para hydrogen differ in
- a) Proton spin
- b) Electron spin
- c) Nuclear charge
- d) Nuclear reaction
Answer: Proton spin
Question: Action of water or dilute mineral acids on metals can give
- a) Monohydrogen
- b) Tritium
- c) Dihydrogen
- d) Trihydrogen
Answer: Dihydrogen
Question: Deuterium resembles hydrogen in chemical properties but reacts
- a) More vigorously than hydrogen
- b) Faster than hydrogen
- c) Slower than hydrogen
- d) Just as hydrogen
Answer: Slower than hydrogen
Question: Spin isomerism is shown by
- a) Dichloro benzene
- b) Hydrogen
- c) Dibasic acid
- d) n-butane
Answer: Hydrogen
Question: Hydrogen can be fused to form helium at
- a) High temperature and high pressure
- b) High temperature and low pressure
- c) Low temperature and high pressure
- d) Low temperature and low pressure
Answer: High temperature and high pressure
Question: What is formed when calcium carbide reacts with heavy water
- a) C2D2
- b) CaD2
- c) Ca2D2O
- d) CD2
Answer: C2D2
Question: Maximum number of hydrogen bonding in H2O is
- a) 1
- b) 2
- c) 3
- d) 4
Answer: 4
Question: In which of the following reaction hydrogen peroxide is a reducing agent?
- a) 2FeCl2+ 2HCl + H2O2→2FeCl3+ 2H2O
- b) Cl2+ H2O2→2HCl + O2
- c) 2HI + H2O2→2H2O + I2
- d) H2SO3+ H2O2→H2SO4+ H2O
Answer: Cl2+ H2O2→2HCl + O2
Question: There is a sample of 10 volume of hydrogen peroxide solution. Calculate its strength.
- a) 3.00%
- b) 4.045%
- c) 2.509%
- d) 3.035%
Answer: 3.035%
Question: In lab H2O2 is prepared by
- a) Cold H2SO4+ BaO2
- b) HCl + BaO2
- c) Conc. H2SO4+ Na2O2
- d) H2+ O2
Answer: Cold H2SO4+ BaO2
Question: H2O2 acts as an oxidising agent in
- a) Acidic medium only
- b) Alkaline medium only
- c) Neutral medium only
- d) Acidic and alkaline medium
Answer: Acidic and alkaline medium
Question: Hydrogen peroxide is reduced by
- a) Ozone
- b) Barium peroxide
- c) Acidic solution of KMnO4
- d) Lead sulphide suspension
Answer: Lead sulphide suspension
Question: The volume of oxygen liberated from 15 ml of 20 volume H2O2 is
- a) 250 ml
- b) 300 ml
- c) 150 ml
- d) 200 ml
Answer: 300 ml
| NEET Chemistry Alcohols Phenols and Ethers MCQs Set A |
| NEET Chemistry Alcohols Phenols and Ethers MCQs Set B |
| NEET Chemistry Aldehydes Ketones and Carboxylic Acids MCQs Set A |
| NEET Chemistry Aldehydes Ketones and Carboxylic Acids MCQs Set B |
| NEET UG Chemistry Biomolecule MCQs |
| NEET UG Chemistry Chemical Bonding MCQs |
| NEET Chemistry Chemical Bonding and Molecular Structure MCQs Set A |
| NEET Chemistry Chemical Bonding and Molecular Structure MCQs Set B |
| NEET Chemistry Chemical Kinetics MCQs Set A |
| NEET Chemistry Chemical Kinetics MCQs Set B |
| NEET UG Chemistry Chemical Kinetics MCQs |
| NEET UG Chemistry Chemical Thermodynamics MCQs |
| NEET Chemistry Chemistry In Everyday Life MCQs Set A |
| NEET Chemistry Chemistry In Everyday Life MCQs Set B |
| NEET UG Chemistry in Everyday Life MCQs |
| NEET UG Chemistry Classification of Elements MCQs |
| NEET Chemistry Classification Of Elements and Periodicity In Properties MCQs Set A |
| NEET Chemistry Classification Of Elements and Periodicity In Properties MCQs Set B |
| NEET UG Chemistry D and F Block Elements MCQs |
| NEET Chemistry Electrochemistry MCQs Set A |
| NEET Chemistry Electrochemistry MCQs Set B |
| NEET Chemistry Electrochemistry MCQs Set C |
| NEET Chemistry Environmental Chemistry MCQs Set A |
| NEET Chemistry Environmental Chemistry MCQs Set B |
| NEET UG Chemistry Environmental Chemistry MCQs |
| NEET Chemistry Equilibrium MCQs Set A |
| NEET Chemistry Equilibrium MCQs Set B |
| NEET Chemistry Equilibrium MCQs Set C |
| NEET UG Chemistry Equilibrium MCQs |
| NEET Chemistry General Principles and Processes Of Isolation Of Elements MCQs Set A |
| NEET Chemistry General Principles and Processes Of Isolation Of Elements MCQs Set B |
| NEET Chemistry Haloalkanes and Haloarenes MCQs Set A |
| NEET Chemistry Haloalkanes and Haloarenes MCQs Set B |
| NEET Chemistry Hydrocarbons MCQs Set A |
| NEET Chemistry Hydrocarbons MCQs Set B |
| NEET Chemistry Hydrocarbons MCQs Set C |
| NEET UG Chemistry Hydrocarbons MCQs |
| NEET Chemistry Hydrogen MCQs Set A |
| NEET Chemistry Hydrogen MCQs Set B |
| NEET UG Chemistry Hydrogen MCQs |
| NEET UG Chemistry Isolation of Metals MCQs |
| NEET UG Chemistry Organic Chemistry MCQs |
| NEET UG Chemistry Organic Compounds Containing Halogens MCQs |
| NEET UG Chemistry Organic Compound Containing Nitrogen MCQs |
| NEET UG Chemistry Organic Compounds MCQs |
| NEET UG Chemistry Organic Compounds Containing Oxygen MCQs |
| NEET UG Chemistry P Block Elements MCQs |
| NEET UG Chemistry Practicals MCQs |
| NEET UG Chemistry Redox Reactions and Electrochemistry MCQs |
| NEET UG Chemistry S Block Elements MCQs |
| NEET Chemistry Solutions MCQs Set A |
| NEET Chemistry Solutions MCQs Set B |
| NEET UG Chemistry Solutions MCQs |
| NEET Chemistry Some Basic Concepts Of Chemistry MCQs Set A |
| NEET Chemistry Some Basic Concepts Of Chemistry MCQs Set B |
| NEET UG Chemistry Some Basic Concepts MCQs |
| NEET Chemistry States Of Matter MCQs Set A |
| NEET Chemistry States Of Matter MCQs Set B |
| NEET UG Chemistry States of Matter MCQs |
| NEET Chemistry Structure Of Atom MCQs Set A |
| NEET Chemistry Structure Of Atom MCQs Set B |
| NEET UG Chemistry Structure of Atom MCQs |
| NEET Chemistry Surface Chemistry MCQs Set A |
| NEET UG Chemistry Surface Chemistry MCQs |
| NEET Chemistry The D and F Block Elements MCQs Set A |
| NEET Chemistry The D and F Block Elements MCQs Set B |
| NEET Chemistry The P Block Elements MCQs Set A |
| NEET Chemistry The P Block Elements MCQs Set B |
| NEET Chemistry The P Block Elements MCQs Set C |
Important Practice Resources for NEET Chemistry Advanced Study Material
MCQs for Hydrogen Chemistry Full Syllabus
Students can use these MCQs for Hydrogen to quickly test their knowledge of the chapter. These multiple-choice questions have been designed as per the latest syllabus for Full Syllabus Chemistry released by NEET. Our expert teachers suggest that you should practice daily and solving these objective questions of Hydrogen to understand the important concepts and better marks in your school tests.
Hydrogen NCERT Based Objective Questions
Our expert teachers have designed these Chemistry MCQs based on the official NCERT book for Full Syllabus. We have identified all questions from the most important topics that are always asked in exams. After solving these, please compare your choices with our provided answers. For better understanding of Hydrogen, you should also refer to our NCERT solutions for Full Syllabus Chemistry created by our team.
Online Practice and Revision for Hydrogen Chemistry
To prepare for your exams you should also take the Full Syllabus Chemistry MCQ Test for this chapter on our website. This will help you improve your speed and accuracy and its also free for you. Regular revision of these Chemistry topics will make you an expert in all important chapters of your course.
You can get most exhaustive NEET Chemistry Hydrogen MCQs Set B for free on StudiesToday.com. These MCQs for Full Syllabus Chemistry are updated for the 2025-26 academic session as per NEET examination standards.
Yes, our NEET Chemistry Hydrogen MCQs Set B include the latest type of questions, such as Assertion-Reasoning and Case-based MCQs. 50% of the NEET paper is now competency-based.
By solving our NEET Chemistry Hydrogen MCQs Set B, Full Syllabus students can improve their accuracy and speed which is important as objective questions provide a chance to secure 100% marks in the Chemistry.
Yes, Chemistry MCQs for Full Syllabus have answer key and brief explanations to help students understand logic behind the correct option as its important for 2026 competency-focused NEET exams.
Yes, you can also access online interactive tests for NEET Chemistry Hydrogen MCQs Set B on StudiesToday.com as they provide instant answers and score to help you track your progress in Chemistry.

