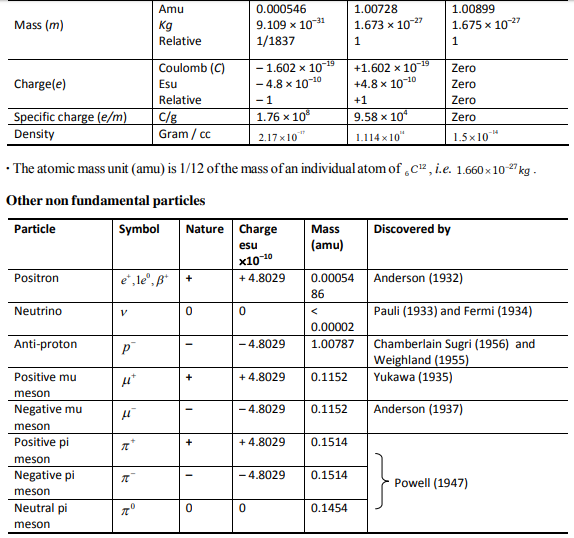
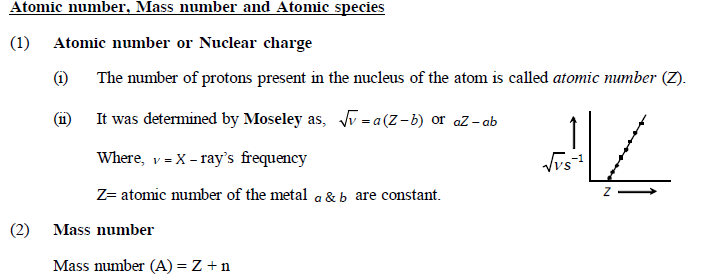
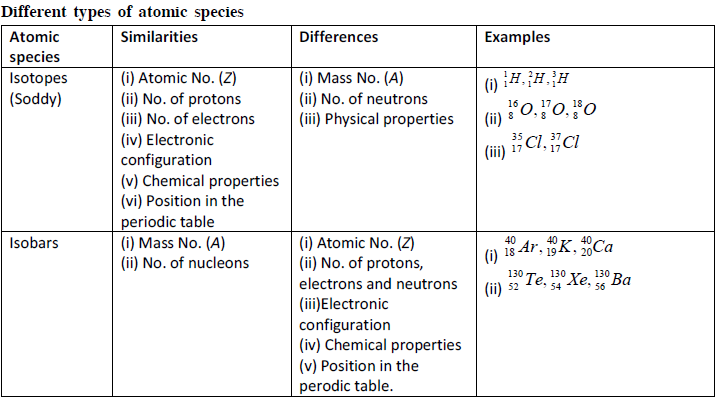
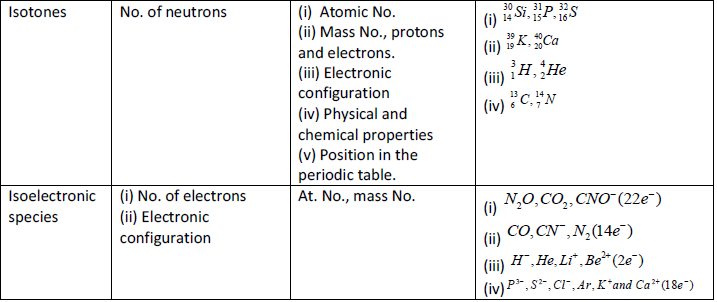
Important Points
Composition of atom
Electron (–1eo)
(1) It was discovered by J.J. Thomson (1897) and is negatively charged particle.
(2) Electron is a component particle of cathode rays.
(3) Cathode rays were discovered by William Crooke’s & J.J. Thomson (1880).
Properties of Cathode rays
(i) Cathode rays travel in straight line.
(ii) Cathode rays produce mechanical effect, as they can rotate the wheel placed in their path.
(iii) Cathode rays consist of negatively charged particles known as electron.
(iv) When cathode rays fall on solids such as Cu, X - rays are produced.
(v) The nature of these rays does not depend upon the nature of gas or the cathode material used in discharge tube.
(vi) The e/m (charge to mass ratio) for cathode rays was found to be the same as that for an e -(-1.76 ´108 coloumb per gm). Thus, the cathode rays are a stream of electrons.
Proton ( H+, p)
(1) Proton was discovered by Goldstein
(2) It is a component particle of anode rays.
Goldstein (1886) used perforated cathode in the discharge tube and repeated Thomson’s
experiment and observed the formation of anode rays. These rays also termed as positive or canal rays.
Properties of anode rays
(i) Anode rays travel in straight line.
(ii) Anode rays are material particles.
(iii) Anode rays are positively charged.
(iv) Anode rays may get deflected by external magnetic field.
(v) Anode rays also affect the photographic plate.
(vi) The e/m ratio of these rays is smaller than that of electrons.
(vii) Unlike cathode rays, their e/m value is dependent upon the nature of the gas taken in the tube.
It is maximum when gas present in the tube is hydrogen.
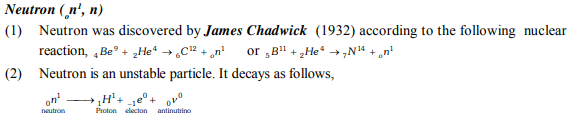
Electromagnetic radiations
(1) Light and other forms of radiant energy propagate without any medium in the space in the form of waves are known as electromagnetic radiations. These waves can be produced by a charged body moving in a magnetic field or a magnet in a electric field. e.g. α - rays, γ - rays, cosmic rays, ordinary light rays etc.
(2) Characteristics
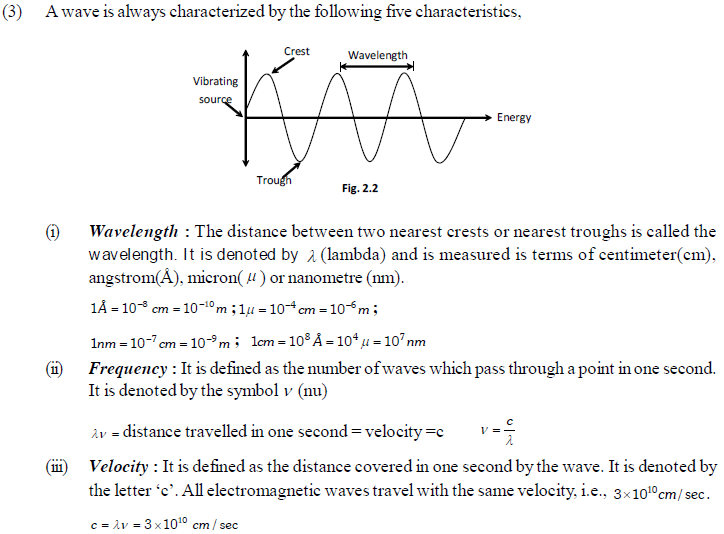
(i) All electromagnetic radiations travel with the velocity of light.
(ii) These consist of electric and magnetic fields components that oscillate in directions perpendicular to each other and perpendicular to the direction in which the wave is travelling.
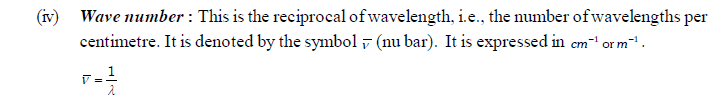
(v) Amplitude : It is defined as the height of the crest or depth of the trough of a wave. It is denoted by the letter ‘A’. It determines the intensity of the radiation.
The arrangement of various types of electromagnetic radiations in the order of their increasing or decreasing wavelengths or frequencies is known as electromagnetic spectrum.
Atomic spectrum - Hydrogen spectrum
Atomic spectrum
Spectrum is the impression produced on a photographic film when the radiation of particular wavelength is (are) analysed through a prism or diffraction grating.
Types of spectrum
(1) Emission spectrum: Spectrum produced by the emitted radiation is known as emission spectrum. This spectrum corresponds to the radiation emitted (energy evolved) when an excited electron returns back to the ground state.
(i) Continuous spectrum: When sunlight is passed through a prism, it gets dispersed into continuous bands of different colours. If the light of an incandescent object resolved through prism or spectroscope, it also gives continuous spectrum of colours.
(ii) Line spectrum: If the radiation’s obtained by the excitation of a substance are analysed with help of a spectroscope a series of thin bright lines of specific colours are obtained.
There is dark space in between two consecutive lines. This type of spectrum is called line spectrum or atomic spectrum..
(2) Absorption spectrum : Spectrum produced by the absorbed radiations is called absorption spectrum.
Hydrogen spectrum
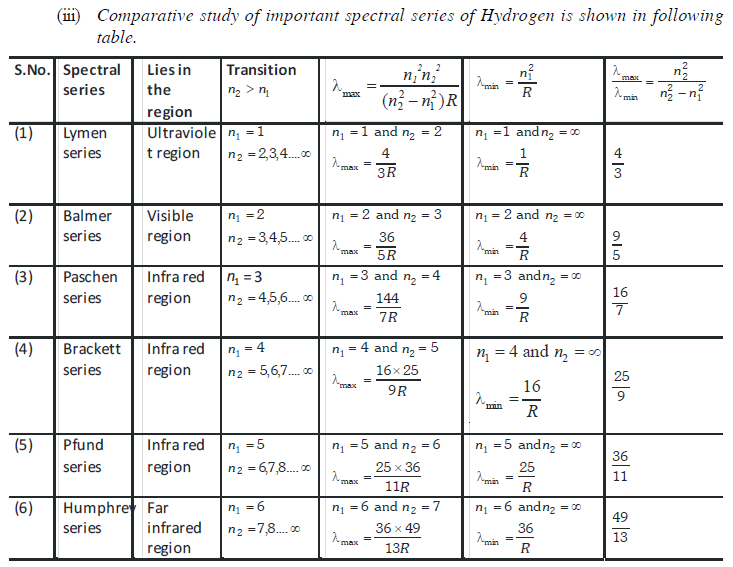
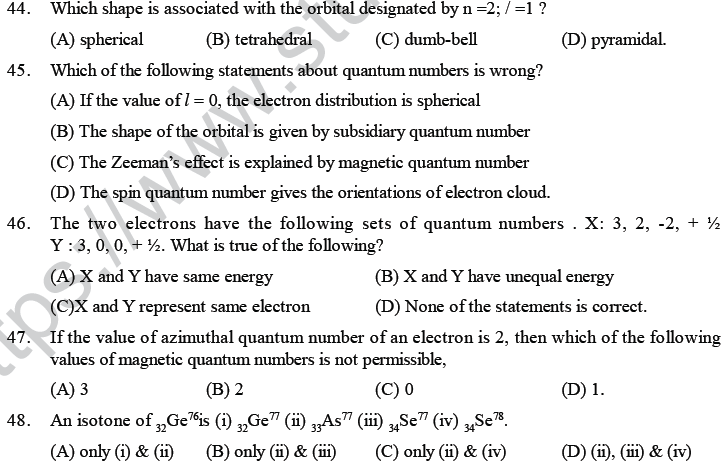
(1) All these lines of H-spectrum have Lyman, Balmer, Paschen, Barckett, Pfund and Humphrey series. These spectral series were named by the name of scientist discovered them.
(2) To evaluate wavelength of various H-lines Ritz introduced the following expression,
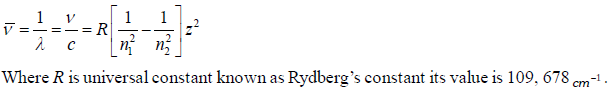
Plum pudding model of Thomson
(1) He suggected that atom is a positively charged sphere having electrons embedded uniformly giving an overall picture of plum pudding.
(2) This model failed to explain the line spectrum of an element and the scattering experiment of Rutherford.
Rutherford’s nuclear model
From the observations of ? ray scattering experiments he concluded that, an atom consists of
(i) Nucleus which is small in size but carries the entire mass i.e. contains all the neutrons and protons.
(ii) Extra nuclear part which contains electrons. This model was similar to the solar system.
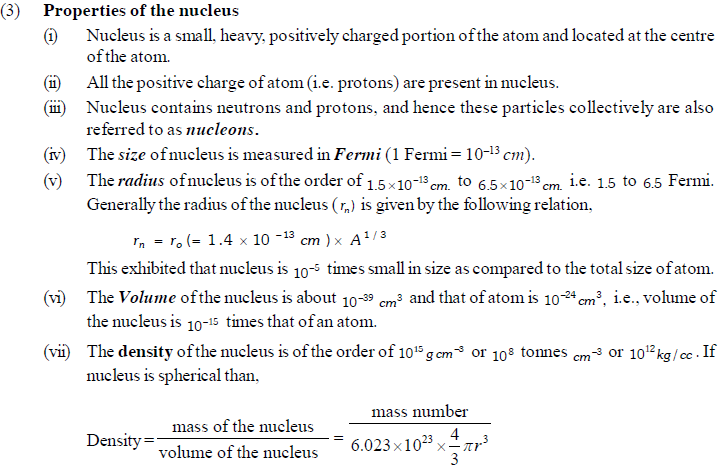

Photoelectric effect
(1) When radiations with certain minimum frequency (ν0 ) strike the surface of a metal, the electrons are ejected from the surface of the metal. This phenomenon is called photoelectric effect and the electrons emitted are called photo-electrons. The current constituted by photoelectrons is known as photoelectric current. This minimum frequency is known as threshold frequency.
(2) The electrons are ejected only if the radiation striking the surface of the metal has at least a minimum frequency called Threshold frequency. The minimum potential at which the plate photoelectric current becomes zero is called stopping potential.
(3) The velocity or kinetic energy of the electron ejected depend upon the frequency of the incident radiation and is independent of its intensity.
(4) The number of photoelectrons ejected is proportional to the intensity of incident radiation.
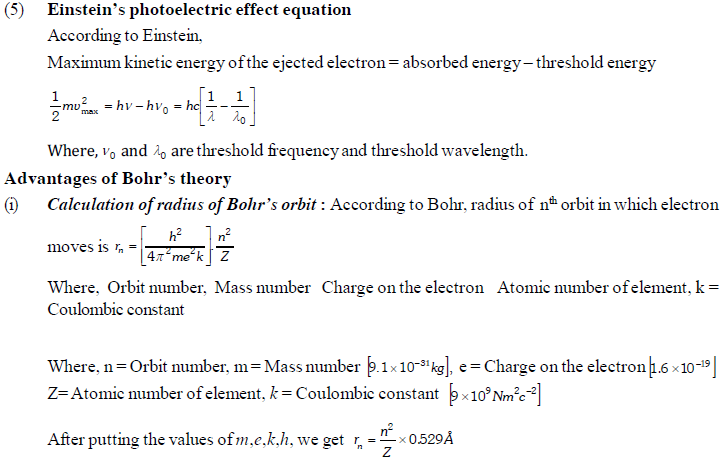
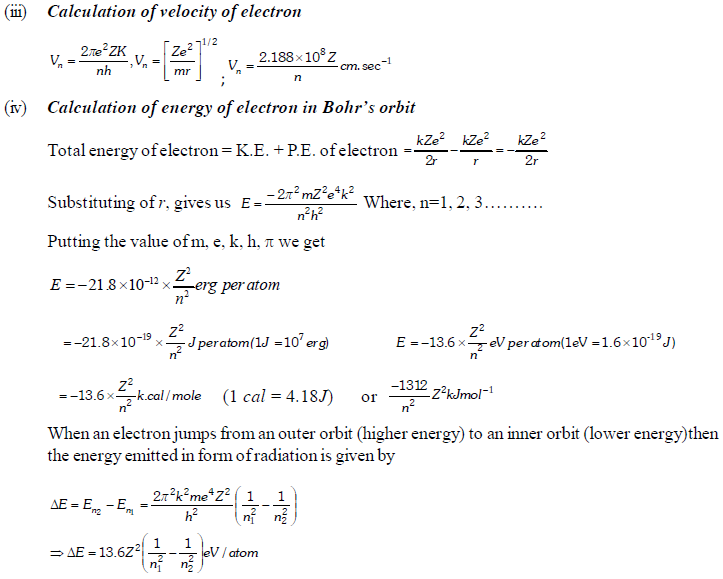
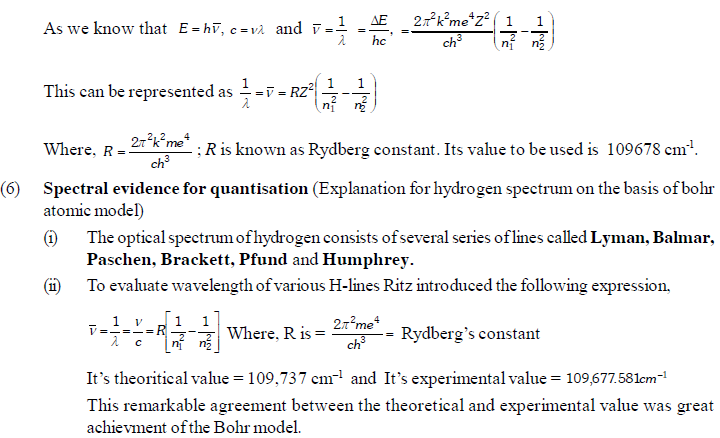
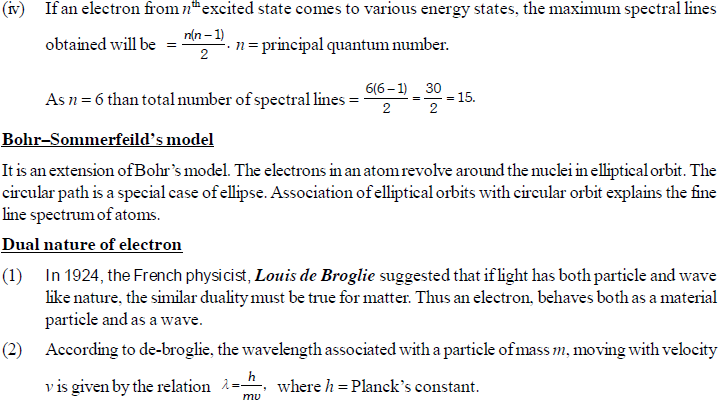
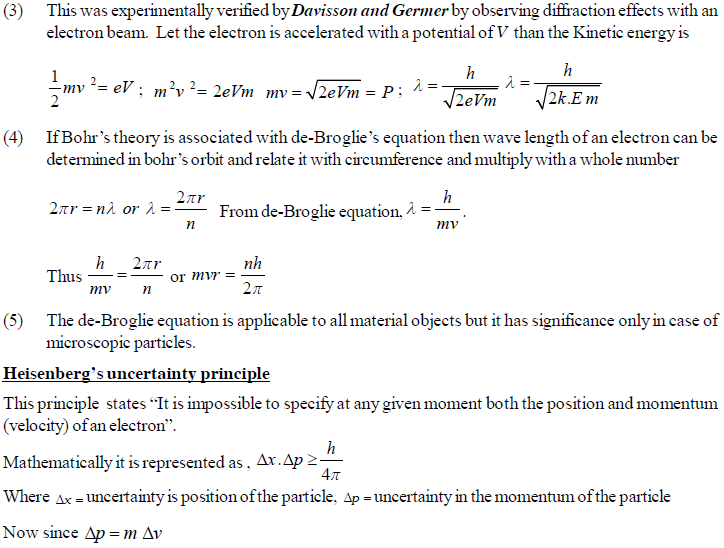

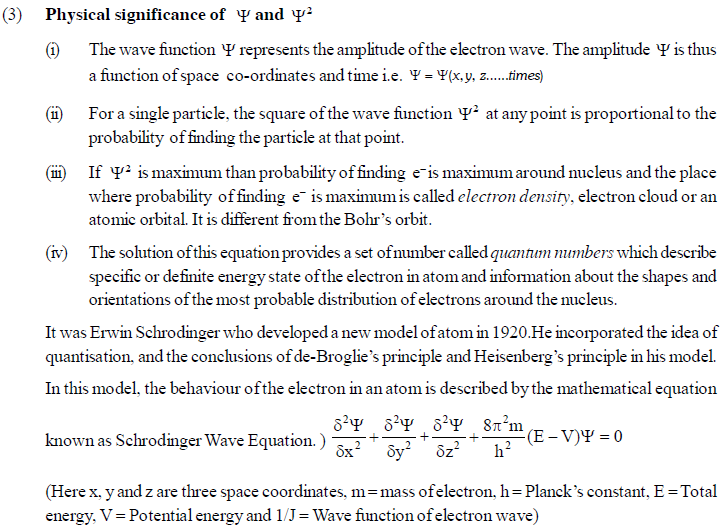
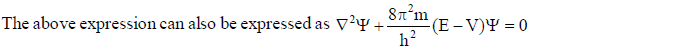
The permitted solutions of Schrodinger equation are known as wave functions which correspond to a definite energy state called orbital. Thus, the discrete Bohr orbits are replaced by orbital’s i.e., three dimensional geometrical olumes where there is maximum probability of locating the electrons.
In simple words, the equation may be interpreted by stating that a body/particle of mass m, potential energy E, velocity v, has wave like characteristics associated with it, with amplitude given by wave function.
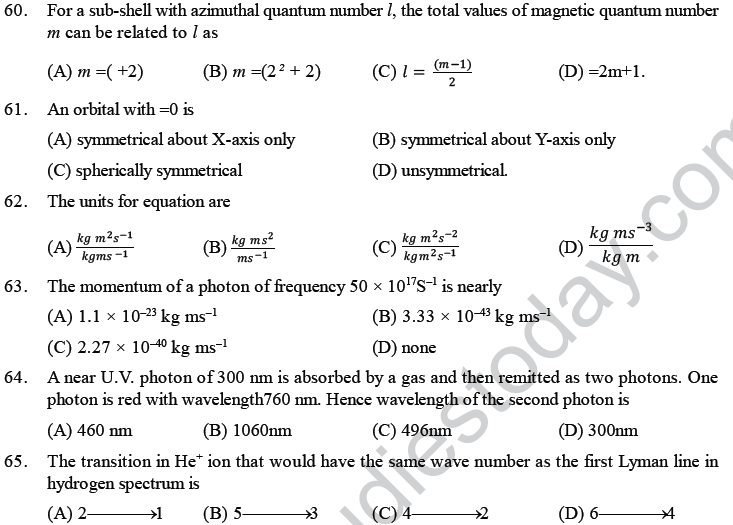
Probability Distribution
In wave mechanics a moving electron is represented by wave function, j. It has on physical significance and refers to the amplitude of electron wave. However, j2 is a significant term and give intensity of electrons. An atomic orbital is a region around the nucleus where there is more probability of intensity of electrons. An orbital is considered as a diffused electron cloud having more density close to the nucleus. The probability of finding an electron in a given volume is understood best in the form of radial probability distribution curves. The probability curves for some orbital’s are given in the figure. The distance of maximum radial probability is radius of an atom. There are two humps for 2p-orbital which means that the 2s electron penetrates a little closer to nucleus. The point at which radial probability becomes zero is known as nodal point.
The radial probability plots for some orbitals are shown in the given figure.
ORBITAL WAVE FUNCTIONS AND SHAPES OF ORBITALS
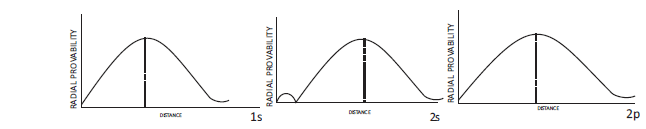
According to wave mechanics, atomic orbitals are described bywave functions known as orbital wave functions. These orbital wave functions can be represented by the product of two wave functions, (i) radial wave function and (ii) angular wave function.
The radial wave function depends upon distance ‘r’ from the nucleus. On the other hand, angular wave function depends upon the direction given by the angles with respect to different co-ordinate axis. It is found that the wave function for s-orbital is independent of angles and, therefore, s-orbitals do not have angular dependence. Thus, all s-orbitals are spherically symmetrical. However, all other types of orbitals (p, d or f) have angular dependence and, therefore, have directional dependence.
Radial Probability Distribution Curves
If we draw a graph between radial wave function, R (radial part of wave functionj) and r (distance from nucleus), we obtain graphs as shown below. These graphs are for ls, 2s and 2p-orbitals of hydrogen atom.
This type of dependence is known as radial dependence. These plots show radial dependence on only one side of the nucleus. These plots do not have any direct physical significance, but are useful in molecular structure because atomic wave functions are’ needed to build molecular wave functions. It is clear from the graph, that in ls radial wave function, j, is positive everywhere, but for other s orbitals i.e., for 2s or 3sorbitals it is positive in some regions and negative in others. It may be noted that +ve and - ve signs have only relative significance. During superposition (in the formation of molecular orbitals) relative signs play a very important part.
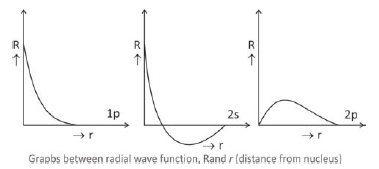
We know that square of the wave function*, R2, represents the probability of finding the electron in a unit volume i.e., probability density. The graphs between square of the radial wave function R2 and r (distance from nucleus) are known as radial probability density graphs. These graphs differ slightly from the earlier graphs as R2 is positive throughout.
M.C.Q.
1. What is wrong about anode rays?
(A) Their e/m ratio is constant
(B) They are deflected by electrical and magnetic field
(C) They are produced by ionisation of molecules of the residual gas
(D) Their elm ratio depends on nature of residual gas.
2. When atoms of the gold sheet are bombarded by a beam of á -particles, only a few á-particles get deflected whereas most of them go straight undeflected. This is because
(A) The force of attraction on α-particles by the oppositely charged electron is not sufficient
(B) The nucleus occupies much smaller volume as compared to the volume of atom
(C) The force of repulsion on fast moving α-particles is very small
(D) The neutrons in the nucleus do not have any effect on α-particles.
3. Which of the following is not a characteristic of Planck’s quantum theory of radiations?
(A) Radiations are associated with energy
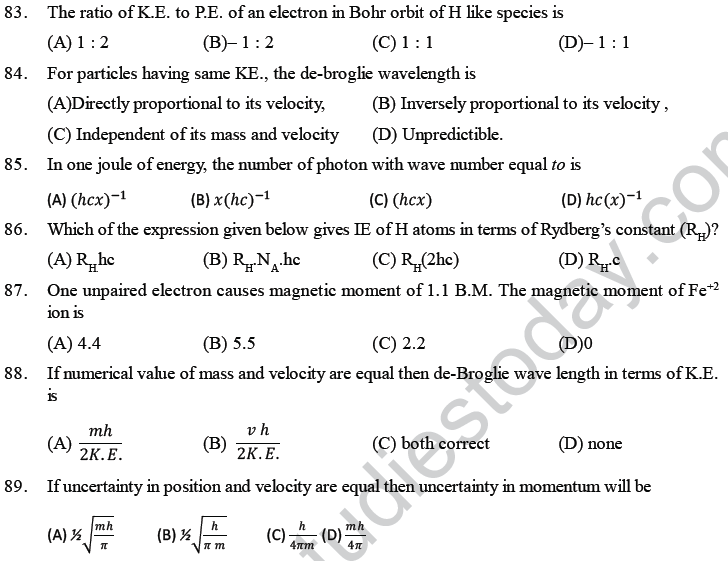
(B) Magnitude of energy associated with a quantum is equal to hv
(C) Radiation energy is neither emitted nor absorbed continuously
(D) A body can emit less or more than a quantum of energy.
4. Which of the following statements is wrong? The probability of finding the electron in px orbital is
(A) Maximum on two opposite sides of the nucleus along x-axis
(B) zero at the nucleus
(C) same on all the sides around the nucleus
(D) zero on the z-axis
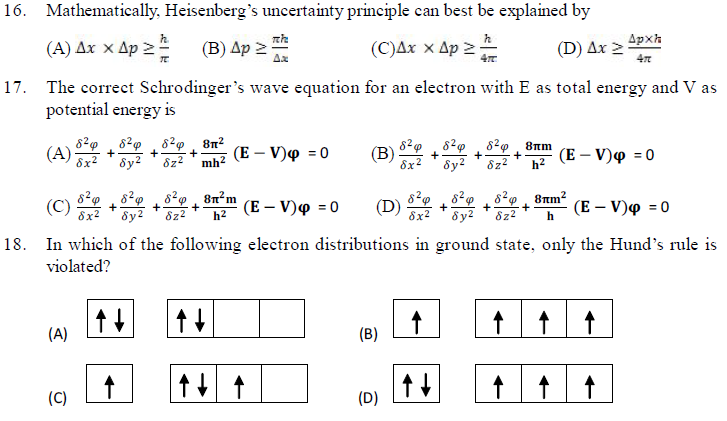
6. The conclusion that every additional electron enters the orbital with lowest possible energy has been drawn from
(A) Pauli’s exclusion principle (B) Hund’s rule
(C) Aufbau principle (D) de-Broglie’s equation.
7. Bohr’s model of atom is not in agreement with
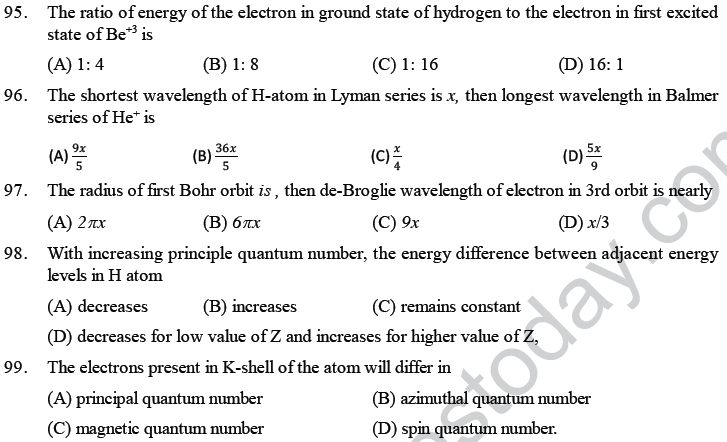
(A) Line spectra of hydrogen atom (B) Pauli’s principle
(C) Planck’s theory (D) Heisenberg’s principle.
8. Which of the following statements is correct?
(A) All electromagnetic radiations do not possess the same velocity
(B) Matter waves are associated with electrical and magnetic fields
(C) Matter waves and electromagnetic radiations are alike
(D) The velocityof matter wave is generally less than that of light
9. Which experimental observation correctly account for the phenomenon?
Experimental observation Phenomenon
(A) X-rays spectra Charge on nucleus
(B) α-particle scattering Quantized electron orbit .
(C) Photoelectric effect The nuclear atom
(D) Emission spectra Quantizationof energy. .
10. In the Schrodinger’s wave equation ø represents
(A) orbit (B) wave function (C) wave (D) radial probability
11. Which of the following gave the idea of a nucleus of the atom?
(A) Oil drop experiment (B) Davissonand Germer’s experiment
(C) α-ray scattering experiment (D) Austen’s mass spectrogram experiment.
12. Cathode rays have same charge to mass ratio as
(A) α-particles (B) β-rays (C) Anode rays (D) Protons
13. Which of the following statements is/are correct?
(A) Isotopes have same number of nucleons
(B) Isobars have same number of protons
(C) Isotones have same number of neutrons
(D) Isobars are atoms of different elements with same isotopic number
15. Rutherford’s experiment established that
(A) inside the atom there are positive centres immersed in sea of electrons
(B) nucleus contains protons, neutrons and mesons
(C) most of the space in an atom is empty
(D) all A, B and C.

21. According to Bohr’s model of the atom, an electron can revolve around the atomic nucleus in a suitable orbit without emitting energy if its orbit
(A) is a perfect circle (B) is a circle with a large radius
(C) houses a whole number of de-Broglie waves
(D) houses odd number of de-Broglie waves.
22. Which of the following concerning Bohr’s model is false?
(A) Predicts that probability of electron near nucleus is more
(B) Angular momentum of electron in H atom
(C) Introduces the idea of stationary states
(D) Explains line spectrum of hydrogen.
24. According to Somerfield’s model, only circular orbit is possible for the electron in
(A) K shell (B) L shell (C) M shell (D) N shell.
25. According to Bohr’s atomic model
(A) electron on H atom can have only certain values of angular momentum
(B) electrons have a particle as well as wave character
(C) atomic spectrum of atom should contain only five lines
(D) all the above statements are correct. .
49. The fundamental particles which arc responsible for keeping nucleons together is
(A) Meson (B) Antiproton (C) Positron (D) Electron. .
50. The positron is as heavy as
(A) electron (B) neutron (C) alpha particle (D) proton.
51. Atoms may be regarded as comprising of protons, electrons and neutrons. If the mass attributed to the neutrons were halved and that attributed to electrons were doubled then atomic mass of would
(A) remain approximately the same (B) be doubled
(C) Approximately be halved (D) be reduced by approximately 25%.
52. How many electrons in an atom with atomic number 105 can have (n +l) = 8 ?
(A) 30 (B) 17 (C) 15 (D) Unpredictable.
53. The size of the nucleus is approximately
(A) 1/100th of the atom (B) 1/1000 th of the atom
(C) 1/10000th of the atom (D) 1/l00000th of the atom.
54. The line spectrum ot two elements is not identical because .
(A) they do not have same number of neutrons
(B) they have dissimilar mass number
(C) they have different energy level schemes
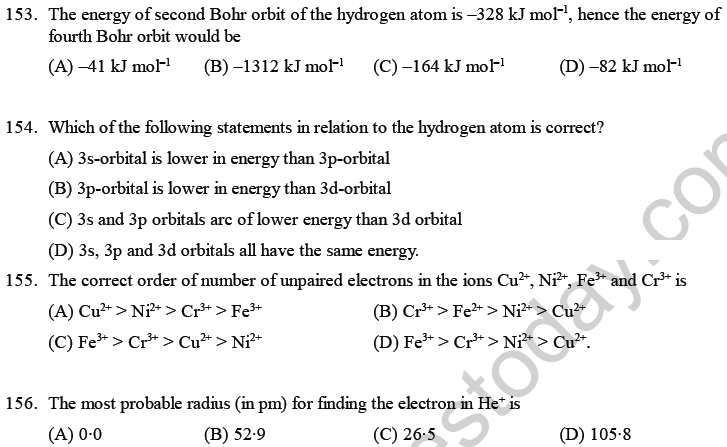
(D) they have different number of valence electrons.
55. Bohr’s atomic model can explain the spectrum of
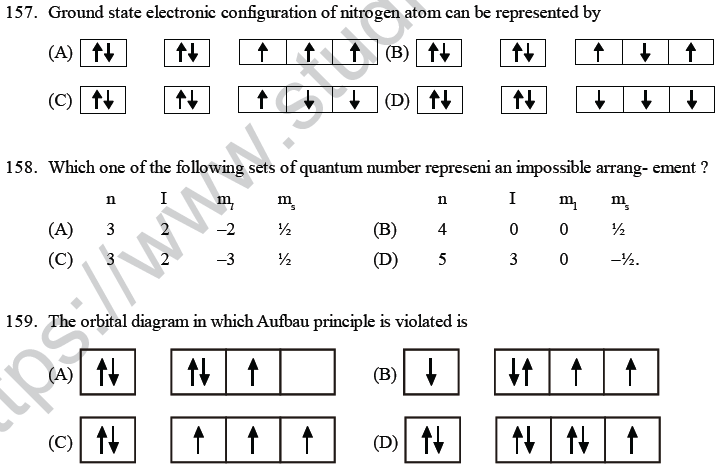
(A) hydrogen atoms only
(B) atoms or ions which are uni electron
(C) atoms or ions which have only two electrons
(D) hydrogen molecule.
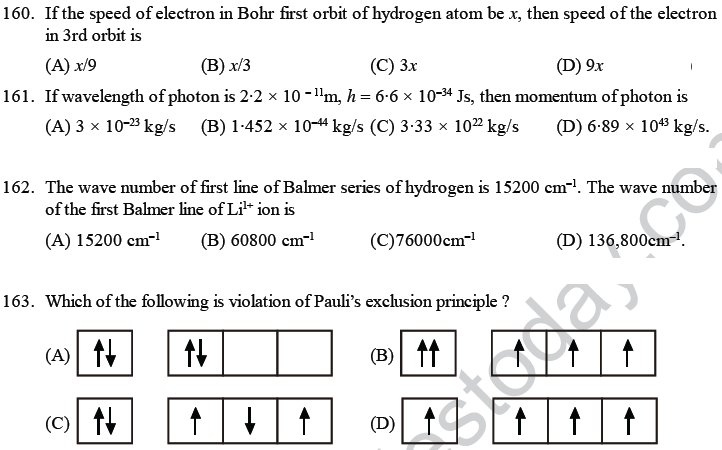
56. The electronic configuration of a dipositive ion M+2 is 2, 8, 14 and its mass number is 56. The number of neutrons present is
(A) 32 (B) 42 (C) 30 (D) 34.
58. An atom has 2 K, 8 L, 11 M, 2 N electrons, the total number of s-electrons will be
(A) 6 (B) 8 (C) 10 (D) 4.
59. In an atom with 2K, 8L, 11M and 2N electrons the number of electrons with m = 0 ; s = + ½ are
(A) 2 (B) 7 (C) 8 (D) 16.




100. The ratio of the ionisation energy of H and Be3+ is_______
(A) 1: 1 (B) 1: 3 (C) 1 : 9 (D) 1: 16.
101. The maximum number of electrons in a subshell for which l = 3 is
(A) 14 (B) 10 (C) 8 (D) 4.
102. The number of electrons in the M shell of the clement with atomic number 24 is
(A) 24 (B) 12 (C) 13 (D) 8.
103. Sodium chloride imparts a yellow colour to the Bunsen flame. This can be interpreted due to the
(A) low ionization energy of sodium
(B) sublimation of metallic sodium to give yellow vapour
(C) emission of excess energy absorbed as a radiation in the visible region
(D) photosensitivity of sodium.
104. The exact path of electron 2p-orbital cannot be determined.” The above statement is based upon
(A) Hund’s Rule (B) Bohr’s Rule (C) Uncertainty principle (D) Aufbau principle.
105. The maximum number of electrons in a subshell is given by the expression
(A) 4l - 2 (B) 4l + 2 (C) 2l + 1 (D) 2n2.
106. If r is the radius of first orbit, the radius of nth orbit of the H atom will be
(A) r n2 (B) rn (C) r/n (D) r2n2
107. The energy of hydrogen atom in its ground state is -13·6 eV. The energy of the level corresponding to the quantum number n = 5 is
(A) “0·54 eV (B) “5·40 eV (C) “0·85 eV (D) “2·72 eV.
108. At 200°C hydrogen molecules have velocity 105 cm sec–1. The de-Broglie wavelength in this case is approximately
(A) 2 Å (B) 1000 Å (C) 100 Å (D) 10 Å.
109. The number of electrons in 3d shell for element with atomic number 26 is
(A) 4 (B) 6 (C) 8 (D) 10.
110. In a set of degenerate orbitals the electrons distribute themselves to retain similar spins as far as possible. This statement is attributed to
(A) Pauli’s exclusion principle (B) Aufbau principle
(C) Hund’s Rule (D) Slater rules.
131. The third line of the Balmer series. in the emission spectrum of the hydrogen atom, is due to the transition from the
(A) fourth Bohr orbit to the first Bohr orbit (B) fifth Bohr orbit to the second Bohr orbit
(C) sixth Bohr orbit to the third Bohr orbit (D) seventh Bohr orbit to the third Bohr orbit
132. The highest number of unpaired electrons are w present in
(A) Fe° (B) Fe4+ (C) Fe2+ (D) Fe3+.
133. Rutherford’s atomic model suggests the existence
(A) Atom (B) Nucleus (C) α-particle (D) Mesons
134. Which is not true with respect to cathode rays?
(A) A stream of electrons (B) Charged particles
(C) Move with speed as that of light (D) Can be deflected by magnetic fields
168. Radial nodes present in 3s and 2p -orbitals are respectively
(A) 0, 2 (B) 2, 0 (C) 2, 1 (D) 1,2.
169. Rutherford’s experiment, which established the nuclear model of the atom, used a beam of
(A) β - particles, which impinged on a metal foil and got absorbed
(B) γ - rays, which impinged on a metal foil and ejected electrons
(C) helium atoms, which impinged on a metal foil and got scattered
(D) helium nuclei, which impinged on a metal foil and got scattered

