Read and download the CBSE Class 9 Chemistry Atoms And Molecules Worksheet Set D in PDF format. We have provided exhaustive and printable Class 9 Science worksheets for Chapter 3 Atoms and Molecules, designed by expert teachers. These resources align with the 2025-26 syllabus and examination patterns issued by NCERT, CBSE, and KVS, helping students master all important chapter topics.
Chapter-wise Worksheet for Class 9 Science Chapter 3 Atoms and Molecules
Students of Class 9 should use this Science practice paper to check their understanding of Chapter 3 Atoms and Molecules as it includes essential problems and detailed solutions. Regular self-testing with these will help you achieve higher marks in your school tests and final examinations.
Class 9 Science Chapter 3 Atoms and Molecules Worksheet with Answers
Express each of the following in kilograms
Question: 5.84 × 10⁻³ mg
Answer: 5.84 × 10⁻⁹ kg
Question: 58.34 g
Answer: 5.834 × 10⁻² kg
Question: 0.584 g
Answer: 5.84 × 10⁻⁴ kg
Question: 5.873 × 10⁻²¹ g
Answer: 5.873 × 10⁻²⁴ kg
Write the formulae for the following and calculate the molecular mass for each one of them.
Question: Caustic potash
Answer: KOH
(39 + 16 + 1) = 56 g mol⁻¹
Question: Baking powder
Answer: NaHCO₃
23 + 1 + 12 + (3 × 16) = 84 g mol⁻¹
Question: Limestone
Answer: CaCO₃
40 + 12 + (3 × 16) = 100 g mol⁻¹
Question: Caustic soda
Answer: NaOH
23 + 16 + 1 = 40 g mol⁻¹
Question: Ethanol
Answer: C₂H₅OH = C₂H₆O
(2 × 12) + (6 × 1) + 16 = 46 g mol⁻¹
Question: Common salt
Answer: NaCl
23 + 35.5 = 58.5 g mol⁻¹
Question: Write the cations and anions present (if any) in the following compounds
(a) CH3COONa
(b) NaCl
(c) H2
(d) NH4NO3
Answer:

Question: Which of the following symbols of elements are incorrect? Give their correct symbols
(a) Cobalt CO
(b) Carbon c
(c) Aluminium AL
(d) Helium He
(e) Sodium So
Answer: (a) Incorrect, the correct symbol of cobalt is Co
(b) Incorrect, the correct symbol of carbon is C
(c) Incorrect, the correct symbol of aluminium is Al
(d) Correct (He)
(e) Incorrect, the correct symbol of sodium is Na
Question: Write the molecular formulae of all the compounds that can be formed by the combination of following ions
Cu2+, Na+ , Fe3+ , Cl– , SO2-4 , PO3-4
Answer: CuCl2/ CuSO4/ Cu3(PO4)2
Question: Classify each of the following on the basis of their atomicity.
(a) F2 (b) NO2 (c) N2O (d) C2H6 (e) P4 (f) H2O2 (g) P4O10 (H) O3 (i) HCl (j) CH4 (k) He (l) Ag
Answer: (a) 2 atom (b) 3 atom (c) 3 atom (d) 8 atom (e) 4 atom (f) 4 atom (g) 14 atom (h) 3 atom (i) 2 atom (j) 5 atom (k) 1 atom (noble gases are always monoatomic) (l) Polyatomic (because metals are bound by metallic bond and any measurable quantity of a metal can contain millions of atoms.)
Question: Give the chemical formulae for the following compounds and compute the ratio by mass of the combining elements in each one of them. (You may use appendix-III).
(a) Ammonia
(b) Carbon monoxide
(c) Hydrogen chloride
(d) Aluminium fluoride
(e) Magnesium sulphide
Answer: (a) NH3; ratio N : H = 14 : 3
(b) CO; ratio C : O = 3 : 4
(c) HCl; ratio H : Cl = 1 : 35.5 or 2 : 71
(d) AlF3; ratio Al : F = 9 : 19
(e) MgS; ratio Mg : S = 3 : 4
Question: Does the solubility of a substance change with temperature? Explain with the help of an example.
Answer: Yes, it is a temperature dependent property. The solubility generally, increases with increase in temperature. For example, you can dissolve more sugar in hot water than in cold water
Question: You are provided with a fine white coloured powder which is either sugar or salt.
How would you identify it without tasting?
Answer: On heating the powder, it will char if it is a sugar.
Alternatively, the powder may be dissolved in water and checked for its conduction of electricity. If it conducts, it is a salt.
Question: Calculate the number of moles of magnesium present in a magnesium ribbon weighing 12 g. Molar atomic mass of magnesium is 24g mol–1.
Answer: Number of moles = mass/molar mass = 12 g/24 g = 0.5 mol
Question: Raunak took 5 moles of carbon atoms in a container and Krish also took 5 moles of sodium atoms in another container of same weight. (a) Whose container heavier? (b) Whose container has more number of atoms?
Answer: (a) Mass of sodium atoms carried by Krish = (5×23)g =115g
While mass of carbon atom carried by Raunak = (5×12)g = 60g
Thus, Krish’s container is heavy
(b) Both the bags have same number of atoms as they have same number of moles of atoms.
Question: Verify by calculating that
(a) 5 moles of CO2 and 5 moles of H2O do not have the same mass.
(b) 240 g of calcium and 240 g magnesium elements have a mole ratio of 3:5.
Answer:
(A) CO2 has molar mass = 44g mol–1
5 moles of CO2 have molar mass = 44 × 5= 220 g
H2O has molar mass = 18 g mol–1
5 moles of H2O have mass = 18 × 5 g= 90 g
(B) Number of moles in 240g Ca metal = 240/40 = 6
Number of moles in 240g of Mg metal = 240/24 = 10
Ratio 6: 10 = 3: 5
Question: Calcium chloride when dissolved in water dissociates into its ions according to the following equation.
CaCl2 (aq) → Ca2+ (aq) + 2Cl– (aq)
Calculate the number of ions obtained from CaCl2 when 222 g of it is dissolved in water.
Answer: 1 mole of calcium chloride = 111g
∴ 222g of CaCl2 is equivalent to 2 moles of CaCl2
Since 1 formula unit CaCl2 gives 3 ions, therefore, 1 mol of CaCl2 will give 3 moles of ions 2 moles of CaCl2 would give 3×2 = 6 moles of ions.
No. of ions = No. of moles of ions × Avogadro number
= 6 × 6.022 × 1023
= 36.132 × 1023
= 3.6132 × 1024 ions
Question: Cinnabar (HgS) is a prominent ore of mercury. How many grams of mercury are present in 225 g of pure HgS? Molar mass of Hg and S are 200.6 g mol-1 and 32 g mol-1 respectively.
Answer: Molar mass of HgS = 200.6 + 32 = 232.6 g mol-1
Mass of Hg in 232.6 g of HgS = 200.6 g
Mass of Hg in 225 g of HgS = 200,6/232.6 X 225 = 194.04g
Question: What is the SI prefix for each of the following multiples and submultiples of a unit?
(a) 103 (b) 10-1 (c) 10-2 (d) 10-6 (e) 10-9 (f) 10-12
Answer: (a) kilo (b) deci (c) centi (d) micro (e) nano (f) pico
Question: A sample of vitamin C is known to contain 24 2.58 ×1024 oxygen atoms. How many moles of oxygen atoms are present in the sample?
Answer: 1 mole of oxygen atoms = 23 6.023×1024 atoms
∴ Number or moles of oxygen atoms = 2.58 X 1024/6.022 X 1023 = 4.28 mol 4.28 moles of oxygen atoms.
Question: What is the fraction of the mass of water due to neutrons?
Answer: Mass of one mole (Avogadro Number) of neutrons ~ 1g

There are 8 neutrons in one atom of oxygen
Mass of 8 neutrons = 8/NA
Fraction of mass of water due to neutrons 8/18
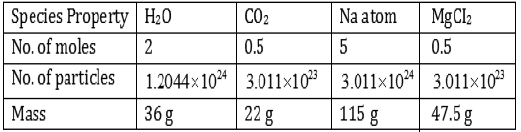
Question: Fill in the missing data in the Table 3.1
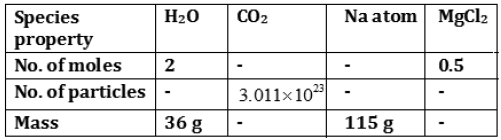
Answer:
Question: The visible universe is estimated to contain 1022 stars. How many moles of stars are present in the visible universe?
Answer: Number of moles of stars = 1022/6.023 X 1023 = 0.0166 moles
Question: Compute the difference in masses of 103 moles each of magnesium atoms and magnesium ions. (Mass of an electron = 9.1×10-31 kg)
Answer: A Mg2+ ion and Mg atom differ by two electrons.
103 moles of Mg2+ and Mg atoms would differ by
103×2 moles of electrons
Mass of 2×103 moles of electrons 2 x 103 x 6.023 x 1023 x 9.1 x 10-31 Kg
2 x 6.022 x 9.1 x 10-5 Kg
109.6004 x 10-5 Kg
1.096 x 10-3 Kg
Question: A gold sample contains 90% of gold and the rest copper. How many atoms of gold are present in one gram of this sample of gold?
Answer: One gram of gold sample will contain 90/100 = 0.9g of gold
Number of moles of gold = mass of gold/atomic mass of gold
= 0.9/197 = 0.0046
One moles of gold contains NA atoms 23 = 6.022×1023
∴ 0.0046 mole of gold will contain 23 = 0.0046×6.022×1023
21 = 2.77×1021
Question: Which has more number of atoms?
(i) 100g of N2
Answer: 100 g of N2 = 100/28, moles
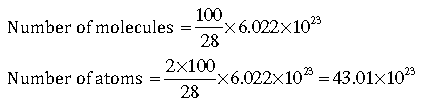
(ii)100 g of NH3
Answer: 100 g of NH3 = 100/17 moles = 100/17 x 6.022 x 1023 molecules
= 100/17 x 6.022 x 1023 x 4 atoms
=141.69×1023
NH3 would have more atoms.
Question: The mass of one steel screw is 4.11g. Find the mass of one mole of these steel screws. Compare this value with the mass of the Earth (5.98 x 1024kg). Which one of the two is heavier and by how many times?
Answer: One mole of screws weigh = 2.475 × 1024 g = 2.475 × 1021 kg
![]()
Mass of earth is 3 2.4×10 times the mass of screws
The earth is 2400 times heavier than one mole of screws.
Question: Compute the number of ions present in 5.85 g of sodium chloride.
Answer: 5.85 g of NaCl = 5.85/58.5 = 0.1 moles
Or 0.1 moles of NaCl particle
Each NaCl particle is equivalent to one Na+ one CI–
⇒ 2 ions
⇒ Total moles of ions = 0.1 × 2
⇒2 moles
No. of ions = 0.2×6.022×1023
23 ⇒1.2042×1023 ions
Question: Compute the difference in masses of one mole each of aluminium atoms and one mole of its ions. (Mass of an electron is 9.1 x 10-28 g). Which one is heavier?
Answer: Mass of 1 mole of aluminium atom = the molar mass of aluminium = 27 g mol-1 An aluminium atom needs to lose three electrons to become an ion, AI3+ For one mole of AI3+ ion, three moles of electrons are to best lost.
The mass of three moles of electrons = 3 x (9.1 x 10-23) x 6.022 x 1023g
= 27.3 x 6.022 x 10-5g
= 164.400 x 10-5g
= 0.00164 g
Molar mass of Al3+ = (27 – 0.00164) g mol-1 = 26.998g mol−1
Difference = 27 – 26.9984 = 0.0016 g
Question: What are ionic and molecular compounds? Give examples.
Answer: Atoms of different elements join together in definite proportions to form molecules of compounds. Examples— water, ammonia, carbondioxide. Compounds composed of metals and non-metals contain charged species. The charged species are known as ions. An ion is a charged particle and can be negatively or positively charged. A negatively charged ion is called an anion and the positively charged ion is called cation. Examples— sodium chloride,
calcium oxide.
Question: A silver ornament of mass ‘m’ gram is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
Answer: Mass of silver = m g
Mass of gold = m/100 g
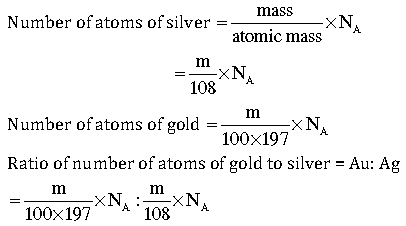
=108:100×197
=108:19700
=1:182.41
Question: A sample of ethane (C2H6) gas has the same mass as 20 1.5×1020 molecules of methane (CH4). How many C2H6 molecules does the sample of gas contain?
Answer: Mass of 1 molecules of CH4 = 16g/NA
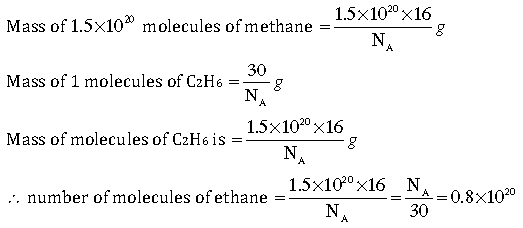
Question: Complete the following crossword puzzle (Fig. 3.1) by using the name of the chemical elements. Use the data given in Table 3.2.
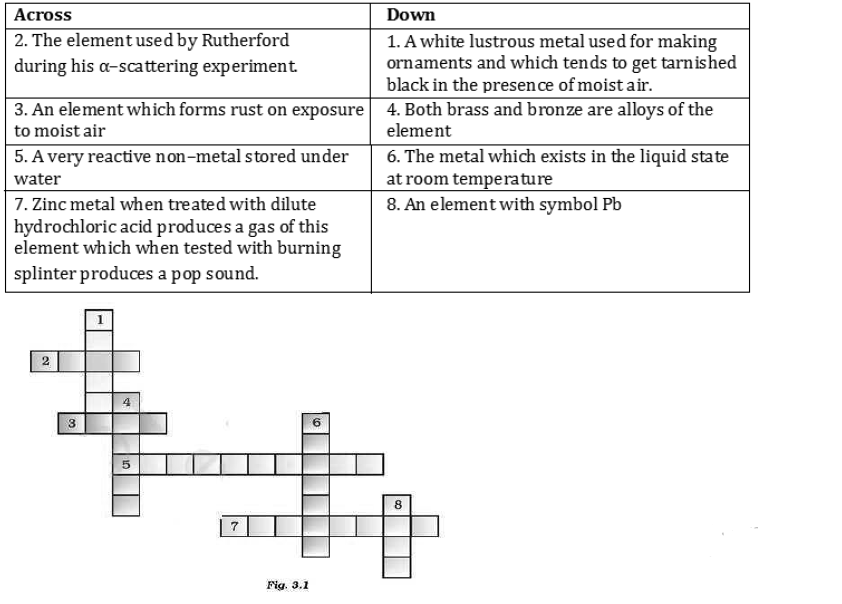
Answer: (1) Silver (2) Gold (3) iron (4) Copper (5) Phosphorus (6) Mercury (7) Hydrogen (8) Lead
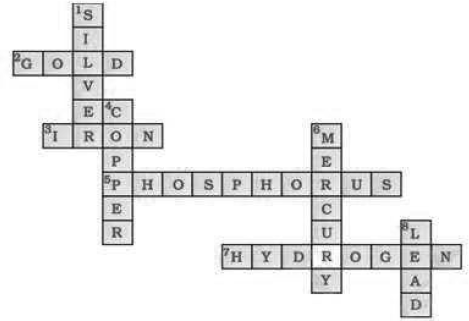
Question: (a) In this crossword puzzle (Fig 3.2), names of 11 elements are hidden Symbols of these are given below. Complete the puzzle. 1. Cl, 2. H, 3. Ar, 4. O, 5. Xe, 6. N, 7. He, 8. F, 9. Kr, 10. Rn, 11. Ne
(b) Identify the total number of inert gases their names and symbols from this cross-word puzzle.
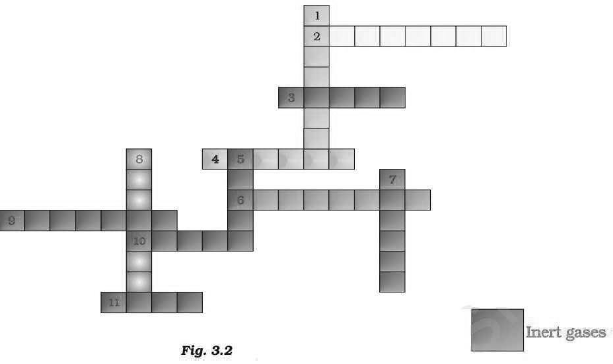
Answer: (a)
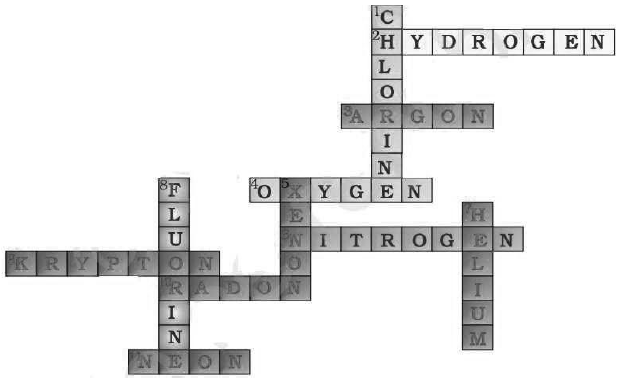
(b) Insert Gases: There are total six insert six inert gases present in this crossword, i.e.
helium (He), Argon (Ar), Xenon (Xe), Krypton (Kr), Radon (Rn) and Neon (Ne).
Question: The difference in the mass of 100 moles each of sodium atoms and sodium ions is 5.48002 g. Compute the mass of an electron.
Answer: A sodium atom and ion, differ by one electron. For 100 moles each of sodium atoms and ions there would be a difference of 100 moles of electrons.
Mass of 100 moles of electrons= 5.48002 g
Mass of 1 mole of electron = (5.48002/100) g
Mass of one electron = 5.48002/100 X 6.022 X 1023 = 9.1 X 10-23 g = 9.1 X 10-31 kg
Question: In photosynthesis, 6 molecules of carbon dioxide combine with an equal number of water molecules through a complex series of reactions to give a molecule of glucose having a molecular formula C6H12O6. How many grams of water would be required to produce 18 g of glucose? Compute the volume of water so consumed assuming the density of water to be 1 g cm-3.
Answer: 6CO2 + 6H2O Chlorophyll/sunlight C6H12 O6 + 6O2 1 mole of glucose needs 6 moles of water 180 g of glucose needs (6 × 18) g of water 1 g of glucose will need 108/180 g of water.
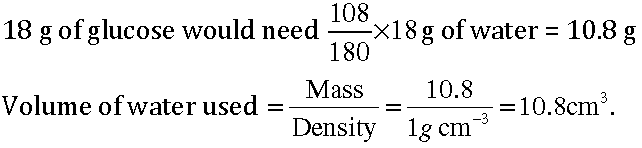
| CBSE Class 9 Chemistry Matter In Our Surrounding Worksheet Set A |
| CBSE Class 9 Chemistry Matter In Our Surrounding Worksheet Set B |
| CBSE Class 9 Chemistry Is Matter Around Us Pure Worksheet Set A |
| CBSE Class 9 Chemistry Is Matter Around Us Pure Worksheet Set B |
| CBSE Class 9 Physics Motion Worksheet Set A |
| CBSE Class 9 Physics Motion Worksheet Set B |
| CBSE Class 9 Physics Gravitation Worksheet Set A |
| CBSE Class 9 Physics Gravitation Worksheet Set B |
| CBSE Class 9 Physics Work And Energy Worksheet Set A |
| CBSE Class 9 Physics Work And Energy Worksheet Set B |
| CBSE Class 9 Physics Work And Energy Worksheet Set C |
| CBSE Class 9 Physics Sound Worksheet Set A |
| CBSE Class 9 Physics Sound Worksheet Set B |
| CBSE Class 9 Physics Sound Worksheet Set C |
| CBSE Class 9 Biology Why Do We Fall Ill Worksheet Set A |
| CBSE Class 9 Biology Why Do We Fall Ill Worksheet Set B |
| CBSE Class 9 Biology Why Do We Fall Ill Worksheet Set C |
| CBSE Class 9 Biology Natural Resources Worksheet Set A |
| CBSE Class 9 Biology Natural Resources Worksheet Set B |
| CBSE Class 9 Biology Natural Resources Worksheet Set C |
Important Practice Resources for Free Printable Worksheets PDF
CBSE Science Class 9 Chapter 3 Atoms and Molecules Worksheet
Students can use the practice questions and answers provided above for Chapter 3 Atoms and Molecules to prepare for their upcoming school tests. This resource is designed by expert teachers as per the latest 2026 syllabus released by CBSE for Class 9. We suggest that Class 9 students solve these questions daily for a strong foundation in Science.
Chapter 3 Atoms and Molecules Solutions & NCERT Alignment
Our expert teachers have referred to the latest NCERT book for Class 9 Science to create these exercises. After solving the questions you should compare your answers with our detailed solutions as they have been designed by expert teachers. You will understand the correct way to write answers for the CBSE exams. You can also see above MCQ questions for Science to cover every important topic in the chapter.
Class 9 Exam Preparation Strategy
Regular practice of this Class 9 Science study material helps you to be familiar with the most regularly asked exam topics. If you find any topic in Chapter 3 Atoms and Molecules difficult then you can refer to our NCERT solutions for Class 9 Science. All revision sheets and printable assignments on studiestoday.com are free and updated to help students get better scores in their school examinations.
You can download the latest chapter-wise printable worksheets for Class 9 Science Chapter Chapter 3 Atoms and Molecules for free from StudiesToday.com. These have been made as per the latest CBSE curriculum for this academic year.
Yes, Class 9 Science worksheets for Chapter Chapter 3 Atoms and Molecules focus on activity-based learning and also competency-style questions. This helps students to apply theoretical knowledge to practical scenarios.
Yes, we have provided solved worksheets for Class 9 Science Chapter Chapter 3 Atoms and Molecules to help students verify their answers instantly.
Yes, our Class 9 Science test sheets are mobile-friendly PDFs and can be printed by teachers for classroom.
For Chapter Chapter 3 Atoms and Molecules, regular practice with our worksheets will improve question-handling speed and help students understand all technical terms and diagrams.

