Read and download the CBSE Class 9 Chemistry Atoms And Molecules Worksheet Set C in PDF format. We have provided exhaustive and printable Class 9 Science worksheets for Chapter 3 Atoms and Molecules, designed by expert teachers. These resources align with the 2025-26 syllabus and examination patterns issued by NCERT, CBSE, and KVS, helping students master all important chapter topics.
Chapter-wise Worksheet for Class 9 Science Chapter 3 Atoms and Molecules
Students of Class 9 should use this Science practice paper to check their understanding of Chapter 3 Atoms and Molecules as it includes essential problems and detailed solutions. Regular self-testing with these will help you achieve higher marks in your school tests and final examinations.
Class 9 Science Chapter 3 Atoms and Molecules Worksheet with Answers
Question: Which of the following correctly represents 360 g of water?
(i) 2 moles of H20
(ii) 20 moles of water
(iii) 6.022 × 1023 molecules of water
(iv) 1.2044×1025 molecules of water
(a) (i)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer: d
Question: Which of the following would weigh the highest?
a) 0.2 mole of sucrose (C₁₂H₂₂O₁₁)
b) 2 moles of CO₂
c) 2 moles of CaCO₃
d) 10 moles of H₂O
Answer: c
Question: The chemical symbol for nitrogen gas is
a) Ni
b) N₂
c) N⁺
d) N
Answer: b
Question: Which of the following contains maximum number of molecules?
a) 1 g CO₂
b) 1 g N₂
c) 1 g H₂
d) 1 g CH₄
Answer: c
Question: The chemical symbol for sodium is
a) So
b) Sd
c) NA
d) Na
Answer: d
Question: Which of the following has maximum number of atoms?
a) 18 g of H₂O
b) 18 g of O₂
c) 18 g of CO₂
d) 18 g of CH₄
Answer: d
Question: Mass of one atom of oxygen is
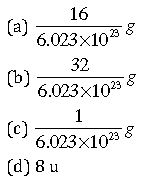
Answer: a
Question: Which of the following statements is not true about an atom?
a) Atoms are not able to exist independently
b) Atoms are the basic units from which molecules and ions are formed
c) Atoms are always neutral in nature
d) Atoms aggregate in large numbers to form the matter that we can see, feel or touch
Answer: a
Question: A change in the physical state can be brought about
a) only when energy is given to the system
b) only when energy is taken out from the system
c) when energy is either given to, or taken out from the system
d) without any energy change
Answer: c
Question: Which of the following represent correct chemical formula? Name it.
a) CaCl
b) BiPO₄
c) NaSO₄
d) NaS
Answer: b
Question: 3.42 g of sucrose are dissolved in 18 g of water in a beaker. The number of oxygen atoms in the solution are
a) 6.68 × 10²³
b) 6.09 × 10²²
c) 6.022 × 10²³
d) 6.022 × 10²¹
Answer: a
Give the formulae of the compounds formed from the following sets of elements
Question: Calcium and fluorine
Answer: CaF₂
Question: Hydrogen and sulphur
Answer: H₂S
Question: Nitrogen and hydrogen
Answer: NH₃
Question: Carbon and chlorine
Answer: CCl₄
Question: Sodium and oxygen
Answer: Na₂O
Question: Carbon and oxygen
Answer: CO, CO₂
Express each of the following in kilograms
Question: 5.84 × 10⁻³ mg
Answer: 5.84 × 10⁻⁹ kg
Question: 58.34 g
Answer: 5.834 × 10⁻² kg
Question: 0.584 g
Answer: 5.84 × 10⁻⁴ kg
Question: 5.873 × 10⁻²¹ g
Answer: 5.873 × 10⁻²⁴ kg
Fill in the blanks
Question: Formula of sodium carbonate is and that of ammonium sulphate is __________.
Answer: Na₂CO₃; (NH₄)₂SO₄
Question: A group of atoms carrying a fixed charge on them is called __________.
Answer: Polyatomic ion
Question: In a chemical reaction, the sum of the masses of the reactants and products remains unchanged. This is called __________.
Answer: Law of conservation of mass
Question: The formula unit mass of Ca₃(PO₄)₂ is __________.
Answer: (3 × atomic mass of Ca) + (2 × atomic mass of P) + (8 × atomic mass of O) = 310
Write the molecular formulae for the following compounds
Question: Copper (II) bromide
Answer: CuBr2
Question: Aluminium (III) nitrate
Answer: Al(NO₃)₃
Question: Calcium (II) phosphate
Answer: Ca₃(PO₄)₂
Question: Iron (III) sulphide
Answer: Fe₂S₃
Question: Mercury (II) chloride
Answer: HgCl₂
Question: Magnesium (II) acetate
Answer: Mg(CH₃COO)₂
State the number of atoms present in each of the following chemical species
Question: CO₃²⁻
Answer: 4
Question: PO₄³⁻
Answer: 5
Question: P₂O₅
Answer: 7
Question: CO
Answer: 2
Find the ratio by mass of the combining elements in the following compounds. (You may use Appendix-III)
Question: CaCO₃
Answer: Ca : C : O₃ = 40 : 12 : (16 × 3) = 40 : 12 : 48 = 10 : 3 : 12
Question: MgCl₂
Answer: Mg : Cl₂ = 24 : (35.55 × 2) = 24 : 71
Question: H₂SO₄
Answer: H₂ : S : O₄ = (1 × 2) : 32 : (16 × 4) = 2 : 32 : 64 = 1 : 16 : 32
Question: C₂H₅OH
Answer: C₂ : H₆ : O = (12 × 2) : (1 × 6) : 16 = 24 : 6 : 16 = 12 : 3 : 8
Question: NH₃
Answer: N : H₃ = 14 : (1 × 3) = 14 : 3
Question: Ca(OH)₂
Answer: Ca : O₂ : H₂ = 40 : (16 × 2) : (1 × 2) = 40 : 32 : 2 = 20 : 16 : 1
Question. Write the value of charge of electron.
Answer: 1.6 × 10–19 coulomb.
Question. Who stated the Law of Constant Proportion
Answer: Joseph Louis Proust stated the Law of Constant Proportion.
Question. What is meant by the term chemical formula?
Answer: The chemical formula of a compound is a symbolic representation of its composition and actual number of atoms in one molecule of a pure substance may be an atom or a compound.
Question.Name the instrument which produces image of the surface of element that shows atoms
Answer: Scanning tunnelling microscope.
Question. Write the symbols of tungsten and iron.
Answer: (i) Tungsten (W) and
(ii) Iron (Fe)
Question.Give the derivation source of symbol of sodium (Na).
Answer: The symbol of ‘Na’ for sodium is derived from its Latin name ‘Natrium’.
Question. What is the atomicity of argon?
Answer: Mono atomic.
Question. What is the latest short form of atomic mass unit?
Answer: The latest short form of atomic mass unit is u,according to IUPAC.
Question. Name any two monatomic atoms.
Answer: Sodium, Aluminium.
Question. Give difference between 2H and H2.
Answer: 2H indicates 2 atoms of hydrogen and H2 indicates one molecule of hydrogen.
Question.Give two examples of triatomic molecules.
Answer: Carbon dioxide (CO2) and water (H2O).
Question. Define valency.
Answer: The combining power of an element to attain the noble gas configuration is called valency. Or, it is defined as number of electrons lost or gained by an atom to acquire noble gas configuration.
Question. Which organisation approves the names of elements all over the world?
Answer: International Union of Pure and Applied Chemistry (IUPAC).
Question. Who introduced the word ‘Mole’?
Answer: ‘Wilhelm Ostwald’ introduced the word ‘Mole’.
Question. What is Avogadro Constant?
Answer: The number of particles present in one mole of any substance is fixed with a value of 6.022 × 1023.
Question. How does an atom exist?
Answer: Atom exists in the form of atom, molecule or ions.
Question. Name the element which is used as the reference for atomic mass.
Answer: Carbon.
Question. Give Latin name of Silver.
Answer: Latin name of Silver is ‘Argentum’.
Question. What is the symbol of the element of molybdenum?
Answer: ‘Mo’ is the symbol of the element of molybdenum.
Question. Give an example in each of the following cases :
(i) a divalent anion
(ii) a trivalent cation
(iii) a mono-valent anion.
Answer: (i) O2– (ii) Fe3+ (iii) I–
Question. What is ion?
Answer: An ion is a charged particle. It can be positive or negative.
Question. What do you mean by symbols of elements?
Answer: Each element is represented by a letter or group of two letters to write the chemical reactions conveniently. It is called symbol.
Question. Who was the first scientist to give the concept of formation of compounds?
Answer: Antoine L. Lavoisier gave the concept of formation of compounds.
Question. Give the symbol of copper, silver, gold, oxygen, zinc.
Answer: Copper - Cu
Silver - Ag
Gold - Au
Oxygen - O
Zinc - Zn
Question. What is the difference between an atom and molecule?
Answer: An atom is the smallest particle of an element which may or may not have independent existence. For example : Na, Al, Fe, etc.
Molecule is the smallest particle of the element o compound which can exist independently. For example : O2, H2, N2, etc.
Question. Name the two laws of chemical combination.
Answer: Law of conservation of mass and law of constant proportions.
Question. Name two elements which have same atomic number.
Answer: Two elements cannot have the same atomic number.
Question. An element has 8 electrons in its valence shell. What is its general name?
Answer: Noble gas.
Question. What is meant by a chemical formula? Give examples.
Answer. A chemical formula of a compound shows its constituent elements and the number of atoms of each combining element, e.g., chemical formula of ammonia is NH3, water is H2O and carbon dioxide is CO2.
Question. Calculate the ratio of the numbers of atoms for magnesium sulphide.
Answer.
Atomic mass of Mg 3/24 = 1/8
Atomic mass of S 4/32 = 1/8
Ratio of atoms in MgS =1/8 : 1/8 or 1 : 1
Question. Why the number of atoms in one mole of hydrogen gas is double the number of atoms in one mole of helium gas? Explain.
Answer. The number of atoms in one mole of hydrogen gas is double the number of atoms in one mole of helium gas because hydrogen molecule is diatomic, i.e., a molecule of hydrogen consists of two atoms of hydrogen, whereas helium is monatomic.
Question. Give one example each of (i) Monovalent cation, (ii) Bivalent cation, (iii) Monovalent anion, (iv) Bivalent anion.
Answer.
(i) K+ or Na+
(ii) Mg+2 or Ca+2
(iii) F– or Cl–
(iv) O2– or S2–
Question. What is Avogadro number?
Answer. The number 6.022 × 1023 is referred to as Avogadro number and is denoted by symbol NA.
Question. What are ionic compounds?
Answer. Ionic compounds are charged particles. Such compounds form by joining or losing or sharing the electron. For example : Sodium chloride is an ionic compound. Its constituent particles are positively charged sodium ion (Na+) and negatively charged chloride ion (Cl–).
Question. State two examples in each case and write their chemical formulae :
(a) Molecules having same kind of atoms only.
(b) Molecules having two different kinds of atoms.
(c) Molecules having three different kinds of atoms.
Answer.
(a) F2, Cl2, P4, S8
(b) Ammonia (NH3), Sulphur dioxide (SO2), Carbon disulphide (CS2).
(c) Calcium sulphate (CaSO4), Sodium nitrate (NaNO3).
Question. What is the difference between hydrogen chloride and nitrogen molecule formation?
Answer. Hydrogen chloride is molecular compound and formed by the union of different kinds of atoms while nitrogen is diatomic molecule and formed by union of two atoms of same kinds.
Question. Write the meaning of these formulae :
(i) 2O,
(ii) O2,
(iii) O3
Answer.
(i) 2O = Two atoms of oxygen
(ii) O2 = One molecule of oxygen
(iii) O3 = One molecule of ozone
Question. Write the name of the compounds : NaBr, Al2O3, CaCO3.
Answer.
NaBr = Sodium bromide
Al2O3 = Aluminium oxide
CaCO3 = Calcium carbonate
Na+, K+, Al3+ and O2– have 10 electrons each Hence, they are iso-electronic.
Question. Find the number of atoms in the 0.5 mole of C atom.
Answer.
0.5 mole of C atom :
Number of atoms in 1 mole of C atom
= 6.022 × 1023 atoms
Number of atoms in 0.5 mole of C atom
= 6.022 × 1023 × 0.5
= 3.011 × 1023 atoms
Question. Name the scientists whose experimentation established laws of chemical combination. Name the laws also.
Answer.Antoine Laurent Lavoisier and Joseph L. Proust experimented and established two laws of chemical combination.
These laws are :
(i) Law of conservation of mass,
(ii) Law of constant proportions.
Question. Write the postulate given by the Indian philosopher Maharishi Kanad.
Answer. Indian philosopher Maharishi Kanad postulated if we divide matter we will get smaller and smaller particles.He said that a time will come when we come across smallest particles beyond which further division will not be possible.
Question. Sunita calculated molecular mass of S8 molecule and reported it as 64u from the given atomic mass of 32u. But teacher considered her answer as wrong. What is the correct molecular mass of S8? Calculate the number of moles of S8 in 25.6 g of the sample.
Answer.
Molecular mass of S8 = 32 × 8 = 256u
Moles of S8 in 25.6 g sample = 25.6/25 6 = 0.1 mole
Question. What is the mass of (i) 2.5 moles of CO2 and (ii) 1 mole of water?
Answer.
(i) 1 mole of CO2 = Molecular mass expressed in grams
= 1 × 44 g
2.5 moles of CO2 = 2.5 × 44 = 110 g
(ii) Mass of the substance = Moles of substance
× Molecular mass in grams
Mass of water = 1 × 18 g = 18 g
Question. What is the use of mole concept?
Answer. Applications of mole concept :
(i) We can calculate the number of basic particles from the number of moles as the number of moles of a substance is directly proportional to the number of elementary particles.
(ii) One mole of gas occupies 22.4 litres at 273K.
(iii) One mole of any gas occupies the same volume at same pressure and temperature.
(iv) One mole is equal to 6.022 × 1023 atoms. So, we can calculate the absolute masses of atoms and molecules.
Question. Define the term gram atom. How is it related to mole and Avogadro number?
Answer.
The atomic mass of an element expressed in grams is called gram atomic mass. One gram atom of any element contains 6.022 × 1023 atoms of the element. It is equal to one mole of atoms.
One gram atomic mass = 6.022 × 1023 atoms = 1 mole
Question. Ca2P2O7 is the formula of calcium pyrophosphate. Write the formula for ferric pyrophosphate.
Answer.
Valency of calcium is +2. Ca2P2O7 has two calcium atoms. So, calcium have total of +4 charges. Thus,pyrophosphate has a valency of –4. Since ferric ion has a valency of +3, the formula of ferric pyrophosphate is Fe4(P2O7)3.
Question. The mass of any single atom X is 3.05 × 10–22 g. What is its atomic weight? Name the possible element.
Answer.
1 mole = atomic mass
= 6.022 × 1023 atoms
Now, mass of one atom of X = 3.05 × 10–22 g
Mass of 6.022 × 1023 atoms of X
= 3.05 × 10–22 × 6.022 × 1023 g
= 183.7 g
This element could be tungsten.
Question. 50 g of 10% lead nitrate is mixed with 50 g of 10% sodium chloride in a closed vessel. It was found after reaction that 6.83 g of lead chloride was precipitated.
Besides, the reaction mixture contained 90 g water and sodium nitrate. Calculate the amount of sodium nitrate formed.
Answer.
50 g of 10% lead nitrate = 5 g lead nitrate + 45 g water
50 g of 10% sodium chloride = 5 g sodium chloride + 45 g water
Total content before reaction = 5 + 5 + 90 = 100
Total content after reaction = 90 g
Amount of precipitate = 6.83 g
According to law of conservation,
Total mass of reaction mixture = 100 g
Amount of sodium nitrate = 100 – 90 – 6.83 = 3.17 g
Question. Explain the law of multiple proportions.
Answer.
According to law of multiple proportions, when two elements combine to make one or more compounds then the ratio of weights of these element remain in fixed ratio to one another. For example : Hydrogen and oxygen combine to form water (H2O) and hydrogen peroxide (H2O2) under different condition. 2 grams of hydrogen combines with 16 grams of oxygen in case of water while 2 grams of hydrogen combines with 32 grams of oxygen to form hydrogen peroxide. Now, the weights of oxygen combine with a fixed weight of hydrogen in water and hydrogen peroxide respectively are 16 and 32 which are in simple ratio of 16: 32 or 1 : 2.
Question. Explain the form of atoms in a solid.
Answer. A solid element is a cluster of atoms. The property of solid does not depend on a single atom but on cluster of atoms. For example : Diamond and graphite though both are composed of carbon atoms but due to different arrangements of carbon atoms in these.They are different in physical and chemical properties.
| CBSE Class 9 Chemistry Matter In Our Surrounding Worksheet Set A |
| CBSE Class 9 Chemistry Matter In Our Surrounding Worksheet Set B |
| CBSE Class 9 Chemistry Is Matter Around Us Pure Worksheet Set A |
| CBSE Class 9 Chemistry Is Matter Around Us Pure Worksheet Set B |
| CBSE Class 9 Physics Motion Worksheet Set A |
| CBSE Class 9 Physics Motion Worksheet Set B |
| CBSE Class 9 Physics Gravitation Worksheet Set A |
| CBSE Class 9 Physics Gravitation Worksheet Set B |
| CBSE Class 9 Physics Work And Energy Worksheet Set A |
| CBSE Class 9 Physics Work And Energy Worksheet Set B |
| CBSE Class 9 Physics Work And Energy Worksheet Set C |
| CBSE Class 9 Physics Sound Worksheet Set A |
| CBSE Class 9 Physics Sound Worksheet Set B |
| CBSE Class 9 Physics Sound Worksheet Set C |
| CBSE Class 9 Biology Why Do We Fall Ill Worksheet Set A |
| CBSE Class 9 Biology Why Do We Fall Ill Worksheet Set B |
| CBSE Class 9 Biology Why Do We Fall Ill Worksheet Set C |
| CBSE Class 9 Biology Natural Resources Worksheet Set A |
| CBSE Class 9 Biology Natural Resources Worksheet Set B |
| CBSE Class 9 Biology Natural Resources Worksheet Set C |
Important Practice Resources for Free Printable Worksheets PDF
CBSE Science Class 9 Chapter 3 Atoms and Molecules Worksheet
Students can use the practice questions and answers provided above for Chapter 3 Atoms and Molecules to prepare for their upcoming school tests. This resource is designed by expert teachers as per the latest 2026 syllabus released by CBSE for Class 9. We suggest that Class 9 students solve these questions daily for a strong foundation in Science.
Chapter 3 Atoms and Molecules Solutions & NCERT Alignment
Our expert teachers have referred to the latest NCERT book for Class 9 Science to create these exercises. After solving the questions you should compare your answers with our detailed solutions as they have been designed by expert teachers. You will understand the correct way to write answers for the CBSE exams. You can also see above MCQ questions for Science to cover every important topic in the chapter.
Class 9 Exam Preparation Strategy
Regular practice of this Class 9 Science study material helps you to be familiar with the most regularly asked exam topics. If you find any topic in Chapter 3 Atoms and Molecules difficult then you can refer to our NCERT solutions for Class 9 Science. All revision sheets and printable assignments on studiestoday.com are free and updated to help students get better scores in their school examinations.
You can download the latest chapter-wise printable worksheets for Class 9 Science Chapter Chapter 3 Atoms and Molecules for free from StudiesToday.com. These have been made as per the latest CBSE curriculum for this academic year.
Yes, Class 9 Science worksheets for Chapter Chapter 3 Atoms and Molecules focus on activity-based learning and also competency-style questions. This helps students to apply theoretical knowledge to practical scenarios.
Yes, we have provided solved worksheets for Class 9 Science Chapter Chapter 3 Atoms and Molecules to help students verify their answers instantly.
Yes, our Class 9 Science test sheets are mobile-friendly PDFs and can be printed by teachers for classroom.
For Chapter Chapter 3 Atoms and Molecules, regular practice with our worksheets will improve question-handling speed and help students understand all technical terms and diagrams.

