Download the latest CBSE Class 10 Chemistry Acids Bases And Salts Notes Set B in PDF format. These Class 10 Science revision notes are carefully designed by expert teachers to align with the 2025-26 syllabus. These notes are great daily learning and last minute exam preparation and they simplify complex topics and highlight important definitions for Class 10 students.
Chapter-wise Revision Notes for Class 10 Science Chapter 2 Acids Bases and Salts
To secure a higher rank, students should use these Class 10 Science Chapter 2 Acids Bases and Salts notes for quick learning of important concepts. These exam-oriented summaries focus on difficult topics and high-weightage sections helpful in school tests and final examinations.
Chapter 2 Acids Bases and Salts Revision Notes for Class 10 Science
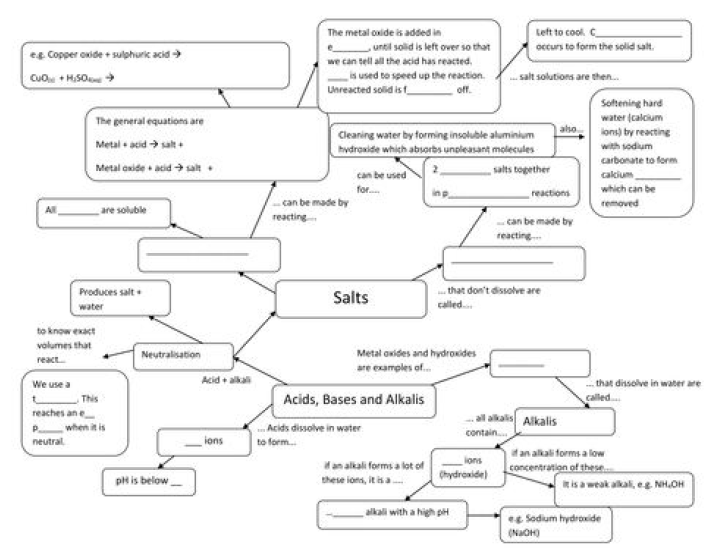
Acids: Substances which turn blue litmus solution red are called acids. Acids are sour in taste Bases: Substances which change red litmus solution blue are called bases. They are bitter in taste.
Mineral Acids: Acids which are obtained from minerals like sulphates, nitrates, chlorides etc. are called mineral acids, e.g., H2SO4(Sulphuric acid), HNO3(Nitric acid) and HCl (Hydrochloric acid).
Organic Acids: Acids which are obtained from plants and animals are called organic acids.e.g. citric acid, ascorbic acid, tartaric acid, lactic acid, acetic acid. Hydronium Ions (H3O + ): They are formed by reaction of H+ (from acid) and H2O. It is because H+ is unstable.
Strong Acids: Acids which dissociate into ions completely are called strong acids. E.g. H2SO4, HCl
Weak Acids: Acids which do not dissociate into ions completely are called weak acids E.g. Citric acid, acetic acid.
Strong bases: The bases in which complete dissociation of hydroxide ions takes place are called Strong Bases. E.g. NaOH, KOH
Weak bases: The bases which do not dissociate completely in aqueous solution to form hydroxide ions are called weak Bases. E.g. NH4OH
Chemical properties of acids
(i) Acids react with active metals to give salt and hydrogen gas.
(ii) Acids react with metal carbonate and metals hydrogen carbonate to give salt, water and carbon dioxide.
(iii) Acids react with bases to give salt and water. This reaction is called neutralization reaction.
iv) Acids react with metals oxides to give salt and water.
Chemical properties of Bases
(i) Reaction with Metals – Certain metals such as Zinc, Aluminum and Tin react with alkali solutions on heating and hydrogen gas is evolved
(ii) Reaction with acids – Bases react with acids to form salt and water
Indicators – Indicators are substances which indicate the acidic or basic nature of the solution by their color change.
Olfactory Indicator: Substances which change their smell when mixed with acid or base are known as Olfactory Indicators. For example; Onion, vanilla etc.
Onion: Paste or juice of onion loses its smell when added with base. It does not change its smell with acid.
Vanilla: The smell of vanilla vanishes with base, but its smell does not vanish with an acid.
Olfactory Indicators are used to ensure the participation of visually impaired students in the laboratory
Universal Indicator: A universal indicator is a mixture of indicators which shows a gradual but wellmarked series of color changes over a very wide range of change in concentration of H+ ion.
pH scale: A scale for measuring hydrogen ion concentration in a solution. The pH of a solution is defined as the negative logarithm of hydrogen ion concentration in moles per litre.
PH =-log [H+] pH =-log [H3O +] where [H+] or [H3O +] represents concentrations of hydrogen ions in solution. The pH of a neutral solution is 7 the pH of an acidic solution is < 7 The pH of a basic solution is> 7
Neutral, Acidic and Basic Salts:
(i) Neutral Salt: Salts produced because of reaction between a strong acid and strong base are neutral in nature. The pH value of such salts is equal to 7, i.e. neutral.
Example: Sodium chloride, Sodium sulphate. Potassium chloride, etc.
(ii) Acidic Salts: Salts which are formed after the reaction between a strong acid and weak base are called Acidic salts. The pH value of acidic salt is lower than 7. For example, Ammonium sulphate, Ammonium chloride, etc.
(iii) Basic Salts: Salts which are formed after the reaction between a weak acid and strong base are called Basic Salts. For example; Sodium carbonate Sodium acetate, etc.
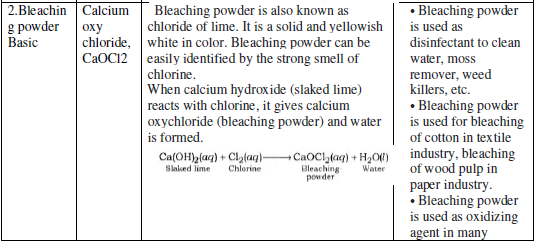
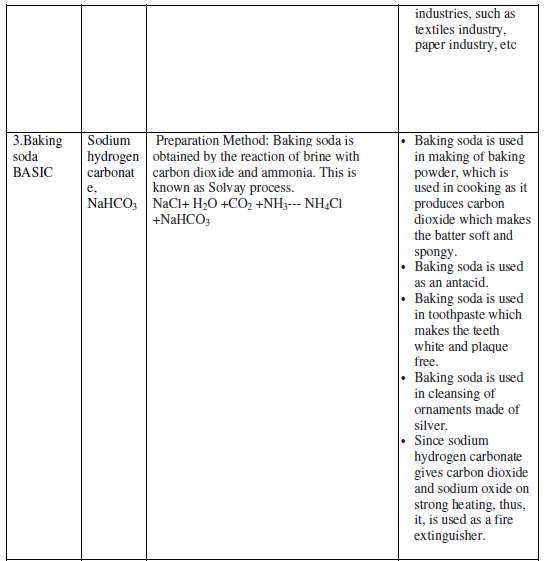
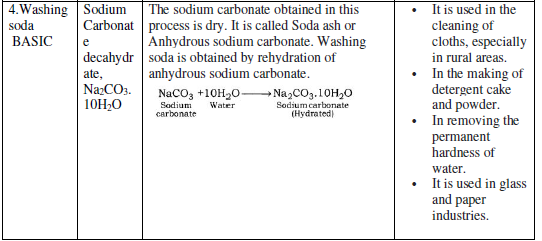
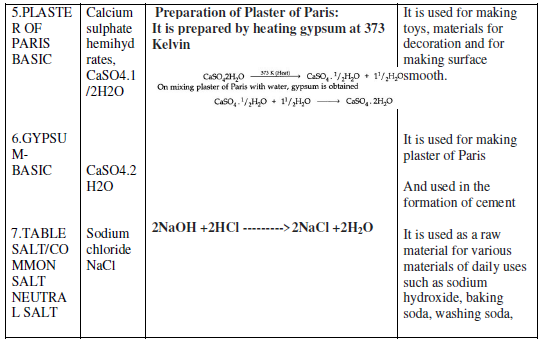
The water of Crystallization: Many salts contain water molecule and are known as Hydrated Salts. The water molecule present in salt is known as Water of crystallization.
Examples:
Copper sulphate pentahydrate (CuSO4.5H2O): Blue color of copper sulphate is due to presence of 5 molecules of water. When copper sulphate is heated, it loses water molecules and turns: into grey – white colour, which is known as anhydrous copper sulphate. After adding water, anhydrous copper sulphate becomes blue again.
EQUATIONS OF ACIDS, BASES AND SALTS
1. Acid + Metal → Salt + Hydrogen gas- H2SO4+Zn → ZnSO4+H2
2. Base+ Metal → Salt + Hydrogen gas – Na2ZnO2 + H2 → 2NaOH + Zn
3. Salt + Water → Base + Acid – NaCl (aq) + H2O (l) → NaOH (aq) +HCl (aq)
4. Acids give hydronium ions in water- HCl+H2O —>H3O+ + Cl
5. Bases generate OH- ions in water NaOH(s)+H2O → Na+ (aq)+OH– (aq)
| CBSE Class 10 Science Chemical Reactions And Chemical Equations Notes Set A |
| CBSE Class 10 Science Chemical Reactions And Chemical Equations Notes Set B |
| CBSE Class 10 Chemistry Acids Bases And Salts Notes Set A |
| CBSE Class 10 Chemistry Acids Bases And Salts Notes Set B |
| CBSE Class 10 Science Metals And Non Metals Notes Set A |
| CBSE Class 10 Science Metals And Non Metals Notes Set B |
| CBSE Class 10 Science Carbon And Its Compounds Notes Set A |
| CBSE Class 10 Science Carbon And Its Compounds Notes Set B |
| CBSE Class 10 Science Light Reflection And Refraction Notes Set A |
| CBSE Class 10 Science Light Reflection And Refraction Notes Set B |
| CBSE Class 10 Science The Human Eye And The Colourful World Notes Set A |
| CBSE Class 10 Science The Human Eye And The Colourful World Notes Set B |
| CBSE Class 10 Science Electricity Notes |
| CBSE Class 10 Science Magnetic Effect Of Current Notes |
| CBSE Class 10 Science Magnetic Effect of Electric Current Notes |
| CBSE Class 10 Science Michael Faraday Notes |
| CBSE Class 10 Science Our Environment Notes |
| CBSE Class 10 Science Biology Slides |
| CBSE Class 10 Science Full Study Material |
Important Practice Resources for Class 10 Science
CBSE Class 10 Science Chapter 2 Acids Bases and Salts Notes
Students can use these Revision Notes for Chapter 2 Acids Bases and Salts to quickly understand all the main concepts. This study material has been prepared as per the latest CBSE syllabus for Class 10. Our teachers always suggest that Class 10 students read these notes regularly as they are focused on the most important topics that usually appear in school tests and final exams.
NCERT Based Chapter 2 Acids Bases and Salts Summary
Our expert team has used the official NCERT book for Class 10 Science to design these notes. These are the notes that definitely you for your current academic year. After reading the chapter summary, you should also refer to our NCERT solutions for Class 10. Always compare your understanding with our teacher prepared answers as they will help you build a very strong base in Science.
Chapter 2 Acids Bases and Salts Complete Revision and Practice
To prepare very well for y our exams, students should also solve the MCQ questions and practice worksheets provided on this page. These extra solved questions will help you to check if you have understood all the concepts of Chapter 2 Acids Bases and Salts. All study material on studiestoday.com is free and updated according to the latest Science exam patterns. Using these revision notes daily will help you feel more confident and get better marks in your exams.
You can download the teacher prepared revision notes for CBSE Class 10 Chemistry Acids Bases And Salts Notes Set B from StudiesToday.com. These notes are designed as per 2025-26 academic session to help Class 10 students get the best study material for Science.
Yes, our CBSE Class 10 Chemistry Acids Bases And Salts Notes Set B include 50% competency-based questions with focus on core logic, keyword definitions, and the practical application of Science principles which is important for getting more marks in 2026 CBSE exams.
Yes, our CBSE Class 10 Chemistry Acids Bases And Salts Notes Set B provide a detailed, topic wise breakdown of the chapter. Fundamental definitions, complex numerical formulas and all topics of CBSE syllabus in Class 10 is covered.
These notes for Science are organized into bullet points and easy-to-read charts. By using CBSE Class 10 Chemistry Acids Bases And Salts Notes Set B, Class 10 students fast revise formulas, key definitions before the exams.
No, all study resources on StudiesToday, including CBSE Class 10 Chemistry Acids Bases And Salts Notes Set B, are available for immediate free download. Class 10 Science study material is available in PDF and can be downloaded on mobile.

