Get the most accurate NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure here. Updated for the 2025-26 academic session, these solutions are based on the latest NCERT textbooks for Class 9 Science. Our expert-created answers for Class 9 Science are available for free download in PDF format.
Detailed Chapter 2 Is Matter Around Us Pure NCERT Solutions for Class 9 Science
For Class 9 students, solving NCERT textbook questions is the most effective way to build a strong conceptual foundation. Our Class 9 Science solutions follow a detailed, step-by-step approach to ensure you understand the logic behind every answer. Practicing these Chapter 2 Is Matter Around Us Pure solutions will improve your exam performance.
Class 9 Science Chapter 2 Is Matter Around Us Pure NCERT Solutions PDF
Class IX Science
Chapter 2 – Is Matter Around Us Pure
Question 1: What is meant by a pure substance?
Answer: A pure substance is the one that consists of a single type of particles, i.e., all constituent particles of the substance have the same chemical nature. Pure substances can be classified as elements or compounds.
Question 2: List the points of differences between homogeneous and heterogeneous mixtures.
Answer: A homogeneous mixture is a mixture having a uniform composition throughout the mixture. For example: salt in water, sugar in water, copper sulphate in water A heterogeneous mixture is a mixture having a non-uniform composition throughout the mixture. For example: sodium chloride and iron fillings, salt and sulphur, oil and water.
Question 3: Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer: A homogeneous mixture is a mixture having a uniform composition throughout the mixture. For example, mixtures of salt in water, sugar in water, copper sulphate in water, iodine in alcohol, alloy, and air have uniform compositions throughout the mixtures.
On the other hand, a heterogeneous mixture is a mixture having a non-uniform composition throughout the mixture. For example, composition of mixtures of sodium chloride and iron fillings, salt and sulphur, oil and water, chalk powder in water, wheat flour in water, milk and water are not uniform throughout the mixtures.
Question 4: How are sol, solution and suspension different from each other?
Answer: Sol is a heterogeneous mixture. In this mixture, the solute particles are so small that they cannot be seen with the naked eye. Also, they seem to be spread uniformly throughout the mixture. The Tyndall effect is observed in this mixture. For example: milk of magnesia, mud.
Solution is a homogeneous mixture. In this mixture, the solute particles dissolve and spread uniformly throughout the mixture. The Tyndall effect is not observed in this mixture. For example: salt in water, sugar in water, iodine in alcohol, alloy.
Suspensions are heterogeneous mixtures. In this mixture, the solute particles are visible to the naked eye, and remain suspended throughout the bulk of the medium. The Tyndall effect is observed in this mixture. For example: chalk powder and water, wheat flour and water.
Question 3: To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer:
Mass of solute (sodium chloride) = 36 g (Given)
Mass of solvent (water) = 100 g (Given)
Then, mass of solution = Mass of solute + Mass of solvent
= (36 + 100) g
= 136 g
Therefore, concentration (mass by mass percentage) of the solution
= Mass of solute / Mass of solvent x 100%
= 36/136 x 100%
= 26.47%
Chapter 2 – Is Matter Around Us Pure
Question 1: How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C), which are miscible with each other?
Answer: A mixture of two miscible liquids having a difference in their boiling points more than 25°C can be separated by the method of distillation. Thus, kerosene and petrol can be separated by distillation.
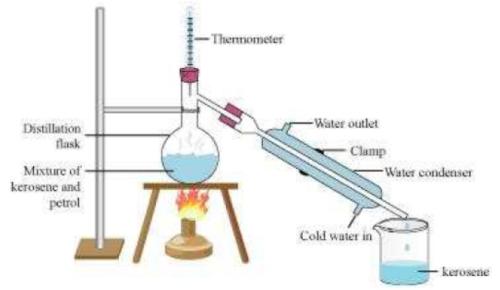
In this method, the mixture of kerosene and petrol is taken in a distillation flask with a thermometer fitted in it. We also need a beaker, a water condenser, and a Bunsen burner. The apparatus is arranged as shown in the above figure. Then, the mixture is heated slowly. The thermometer should be watched simultaneously. Kerosene will vaporize and condense in the water condenser. The condensed kerosene is collected from the condenser outlet, whereas petrol is left behind in the distillation flask.
Question 2: Name the technique to separate
(i) butter from curd
(ii) salt from sea-water
(iii) camphor from salt
Answer: (i) Butter can be separated from curd by centrifugation.
(ii) Salt can be separated from sea-water by evaporation.
(iii) Camphor can be separated from salt by sublimation.
Question 3: What type of mixtures is separated by the technique of crystallization?
Answer: By the technique of crystallization, pure solids are separated from impurities. For example, salt obtained from sea is separated from impurities; crystals of alum (Phitkari) are separated from impure samples.
Chapter 2 – Is Matter Around Us Pure
Question 1: Classify the following as chemical or physical changes:
• Cutting of trees
• Melting of butter in a pan
• Rusting of almirah
• Boiling of water to form steam
• Passing of electric current through water, and water breaking down into hydrogen and oxygen gas
• Dissolving common salt in water
• Making a fruit salad with raw fruits
• Burning of paper and wood
Answer:
• Cutting of trees → Physical change
• Melting of butter in a pan → Physical change
• Rusting of almirah → Chemical change
• Boiling of water to form steam → Physical change
• Passing of electric current through water, and water breaking down into hydrogen and oxygen gas → Chemical change
• Dissolving common salt in water → Physical change
• Making a fruit salad with raw fruits → Physical change
• Burning of paper and wood → Chemical change
Question 2: Try segregating the things around you as pure substances or mixtures.
Answer: Pure substance: Water, salt, sugar
Mixture: Salt water, soil, wood, air, cold drink, rubber, sponge, fog, milk, butter, clothes, food
Chapter 2 – Is Matter Around Us Pure
Question 1: Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride.
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
(f) Oil from water.
(g) Tea leaves from tea.
(h) Iron pins from sand.
(i) Wheat grains from husk.
(j) Fine mud particles suspended in water.
Answer:
(a) Sodium chloride from its solution in water → Evaporation
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride → Sublimation
(c) Small pieces of metal in the engine oil of a car → Centrifugation or filtration or decantation
(d) Different pigments from an extract of flower petals → Chromatography
(e) Butter from curd → Centrifugation
(f) Oil from water → Using separating funnel
(g) Tea leaves from tea → Filtration
(h) Iron pins from sand → Magnetic separation
(i) Wheat grains from husk → Winnowing
(j) Fine mud particles suspended in water → Centrifugation
Question 2: Write the steps you would use for making tea. Use the words: solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer: First, water is taken as a solvent in a saucer pan. This water (solvent) is allowed to boil. During heating, milk and tea leaves are added to the solvent as solutes. They form a solution. Then, the solution is poured through a strainer. The insoluble part of the solution remains on the strainer as residue. Sugar is added tothe filtrate, which dissolves in the filtrate. The resulting solution is the required tea.
Question 4: Explain the following giving examples:
(a) Saturated solution
(b) Pure substance
(c) Colloid
(d) Suspension
Answer:
(a) Saturated solution
A saturated solution is a solution in which the maximum amount of solute has been dissolved at a given temperature. The solution cannot dissolve beyond that amount of solute at that temperature. Any more solute added will settle down at the bottom of the container as a precipitate.
Suppose 500 g of a solvent can dissolve a maximum of 150 g of a particular solute at 40°C. Then, the solution obtained by dissolving 150 g of that solute in 500 g of that solvent at 300 K is said to be a saturated solution at 300 K.
(b) Pure substance
A pure substance is a substance consisting of a single type of particles i.e., all constituent particles of the substance have the same chemical properties.
For example, salt, sugar, water are pure substances.
(c) Colloid
A colloid is a heterogeneous mixture. The size of the solutes in this mixture is so small that they cannot be seen individually with naked eyes, and seems to be distributed uniformly throughout the mixture. The solute particles do not settle down when the mixture is left undisturbed. This means that colloids are quite stable. Colloids cannot be separated by the process of filtration. They can be separated by centrifugation. Colloids show the Tyndall effect. For example, milk, butter, foam, fog, smoke, clouds.
(d) Suspension
Suspensions are heterogeneous mixtures. The solute particles in this mixture remain suspended throughout the bulk of the medium. The particles can be seen with naked eyes. Suspension shows the Tyndall effect. The solute particles settle down when the mixture is left undisturbed. This means that suspensions are unstable. Suspensions can be separated by the method of filtration. For example, mixtures of chalk powder and water, wheat flour and water.
Question 5: Classify each of the following as a homogeneous or heterogeneous mixture.
Soda water, wood, air, soil, vinegar, filtered tea
Answer: Homogeneous mixtures: Soda water, air, vinegar
Heterogeneous mixtures: Wood, soil, filtered tea
Question 6: How would you confirm that a colourless liquid given to you is pure water?
Answer: Every liquid has a characteristic boiling point. Pure water has a boiling point of 100°C (373 K) at 1 atmospheric pressure. If the given colourless liquid boils at even slightly above or below 100°C, then the given liquid is not pure water. It must boil at sharp 100°C. Thus, by observing the boiling point, we can confirm whether a given colourless liquid is pure water or not.
Question 7: Which of the following materials fall in the category of a “pure substance”?
(a) Ice
(b) Milk
(c) Iron
(d) Hydrochloric Acid
(e) Calcium oxide
(f) Mercury
(g) Brick
(h) Wood
(i) Air
Answer: The following materials fall in the category of a “pure substance”:
(a) Ice
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
Question 8: Identify the solutions among the following mixtures:
(a) Soil
(b) Sea water
(c) Air
(d) Coal
(e) Soda water
Answer:
The following mixtures are solutions:
(b) Sea water
(c) Air
(e) Soda water
Question 9: Which of the following will show the “Tyndall effect”?
(a) Salt solution
(b) Milk
(c) Copper sulphate solution
(d) Starch solution
Answer: Milk and starch solution will show the “Tyndall effect”.
Question 10: Classify the following into elements, compounds and mixtures:
(a) Sodium
(b) Soil
(c) Sugar solution
(d) Silver
(e) Calcium carbonate
(f) Tin
(g) Silicon
(h) Coal
(i) Air
(j) Soap
(k) Methane
(l) Carbon dioxide
(m) Blood
Answer:
Elements
(a) Sodium
(d) Silver
(f) Tin
(g) Silicon
Compounds
(e) Calcium carbonate
(k) Methane
(l) Carbon dioxide
Mixtures
(b) Soil
(c) Sugar solution
(h) Coal
(i) Air
(j) Soap
(m) Blood
Question 11: Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of iron
(c) Mixing of iron fillings and sand
(d) Cooking of food
(e) Digestion of food
(f) Freezing of water
(g) Burning of candle
Answer: The following changes are chemical changes:
(a) Growth of a plant
(b) Rusting of iron
(d) Cooking of food
(e) Digestion of food
(g) Burning of candle
Question. In a closed container, 500 g of steam is cooled until all the steam becomes water. The container is then cooled further until all the water becomes ice. Which of the following remains the same during both of these changes?
(a) the mass of the water
(b) the pressure in the container
(c) the temperature of the water
(d) the volume of the water
Answwe : A
Question. Matter may be classified as elements, compounds, or mixtures. Which of the following lists includes only mixtures?
(a) dry ice, alcohol, brass
(b) sea water, milk, air
(c) copper, gasoline, bread
(d) paint, blood, mercury
Answwe : B
Question. A maximum of 16.8 g of compound X can be dissolved in 100 ml of water. In an experiment, different masses of X were added to separate beakers containing varying volumes of water as shown.Arrange these solutions in the DESCENDING order of concentrations.
(a) 4-1-2-3
(b) 4-2-1-3
(c) 1-4-3-2
(d) 1-3-2-4
Answwe : C
Question. The texture, colour and shine of four surfaces A, B, C and D are as shown below. Study the diagram given below and identify which surface will absorb the greatest amount of electromagnetic energy from the Sun?
(a) A
(b) B
(c) C
(d) D
Answwe : B
Question. Figure P below shows a beaker of water being heated directly. However, some liquids like alcohol are heated using a water bath (figure Q). Which of these is NOT likely to be a reason for water baths to be used?
(a) It is safer to use them with inflammable liquids
(b) It allows more uniform heating of the liquid.
(c) It is a faster way of heating a liquid
(d) It is convenient for liquids with low boiling points.
Answwe : C
Question. According to the graph, which of these is the least soluble in water at 20°C.?
(a) CaCl2
(b) KCl
(c) NaCl
(d) LiSO4
Answwe : B
Question. Which option correctly matches the data in the table to the graph.
| KNO | K2SO4 | NaCI | CuSO4 | |
| A. | 1 | 4 | 3 | 2 |
| B. | 3 | 1 | 2 | 4 |
| C. | 2 | 4 | 3 | 1 |
| D. | 1 | 3 | 4 | 2 |
(a) A
(b) B
(c) C
(d) D
Answwe : A
Question. Which of these BEST distinguishes a Physical Change, a Chemical Reaction and a Nuclear Reaction?
(a) A
(b) B
(c) C
(d) D
Answwe : C
Question. If a lighted splint is put into a small sample of a gas and a 'pop' sound is heard, the gas is identified as hydrogen. What is the 'pop' sound due to?
(a) formation of hydrogen molecule
(b) formation of oxygen molecule
(c) combustion of hydrogen
(d) combustion of oxygen
Answwe : C
Question. In cold countries, when snow covers and blocks roads and railway tracks, salt is often put on it. Using that fact, identify the correct graph of ice melting when salt has been put on it:
Answwe : A
Question. Each in the periodic table can appear in gaseous form and will produce a series of spectral lines unique to that element. Thus, scientists can identify what elements are in from the lines they find in the star's spectrum. This type of study is called spectroscopy. Below are the spectral lines of four elements and also an unknown gaseous mixture. Identify the elements in the unknown mixture
(a) Lithium and sodium
(b) Lithium and hydrogen
(c) Helium and hydrogen
(d) Helium and sodium
Answwe : C
Question. 400g each of water, oil and sand are taken and heated from room temperature to 70 deg C on identical Bunsen burners. The time taken for each to reach that temperature is noted. Heating is then stopped and the time taken for each to cool to room temperature is noted. Which of these will be true?
(a) Substances that take more time to get heated will take more time to cool.
(b) Substances that take more time to get heated will take less time to cool.
(c) There is no connection between the time taken to get heated and to cool.
(d) The time taken to get heated depends on the mass, the time taken to cool does not.
Answwe : A
Question. The list given here is known as the Electrochemical Series of metals. A metal lower in the list is more reactive, that is, it will form positive ions more easily, and will also displace a metal that is higher from a solution of the latter metal. Which of the following combinations of metal and salt solutions would result in a coating being formed?
| Metal Plate | Solution | |
| A | Silver | Cobalt Chloride |
| B | Iron | Aluminium Nitrate |
| C | Tin | Zinc Chloride |
| D | Chromium | Lead Bromide |
(a) A
(b) B
(c) C
(d) D
Answwe : D
Question. Two liquids P and Q, and two solids R and S are shown in different undisturbed arrangements below. Study the arrangements and arrange P, Q, R and S in order of INCREASING density.
(a) P < Q < R < S
(b) Q < S < P < R
(c) Q < P < S < R
(d) S < Q < P < R
Answwe : B
Question. Minu found a mineral that was able to scratch a knife blade and it did not split into pieces. Identify the mineral found by her.
Use both the tables and flow chart given on this page to answer the question
(a) Wulfenite
(b) Chalcopyrite
(c) Malachite
(d) Pyrite
Answwe : D
Question. What can we say about the hardness of a material that scratches a knife blade?
Use both the tables and flow chart given on this page to answer the question
(a) It will be 4 or more
(b) It will be 6 or more.
(c) It will be between 6 and 9.
(d) It will be between 4 and 6.
Answwe : B
Question. Which of these minerals has a yellowish shade and cleaves regularly?
Use both the tables and flow chart given on this page to answer the question
(a) Pyrite
(b) Cinnabar
(c) Chalcopyrite
(d) Wulfenite
Answwe : D
Question. A small cube is fixed to the corner of a large cube as shown here.From which of the directions (indicated by the arrows above) would the view of the object be as shown below?
(a) Only 1
(b) 4 and 6
(c) 3 and 5
(d) Only 2
Answwe : A
Question. Till Fredrick Wohler synthesized urea in the laboratory, it was believed that only living organisms could produce organic molecules. According to people who believed this, which of these compounds could NOT be synthesized from chemicals in a laboratory?
(a) Sugar
(b) Water
(c) Salt
(d) Hydrogen gas
Answwe : A
| NCERT Solutions Class 9 Science Chapter 1 Matter in Our Surroundings |
| NCERT Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure |
| NCERT Solutions Class 9 Science Chapter 3 Atoms and Molecules |
| NCERT Solutions Class 9 Science Chapter 4 Structure of the Atom |
| NCERT Solutions Class 9 Science Chapter 5 The Fundamental Unit of Life |
| NCERT Solutions Class 9 Science Chapter 6 Tissues |
| NCERT Solutions Class 9 Science Chapter 7 Motion |
| NCERT Solutions Class 9 Science Chapter 8 Force and Laws of Motion |
| NCERT Solutions Class 9 Science Chapter 9 Gravitation |
| NCERT Solutions Class 9 Science Chapter 10 Work and Energy |
| NCERT Solutions Class 9 Science Chapter 11 Sound |
| NCERT Solutions Class 9 Science Chapter 12 Improvement in Food Resources |
| NCERT Solutions Class 9 Science Chapter 13 Why Do We Fall Ill |
| NCERT Solutions Class 9 Science Chapter 14 Natural Resources |
| NCERT Solutions Class 9 Science Chapter 7 Diversity in Living Organisms |
Important Practice Resources for Class 9 Science
NCERT Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure
Students can now access the NCERT Solutions for Chapter 2 Is Matter Around Us Pure prepared by teachers on our website. These solutions cover all questions in exercise in your Class 9 Science textbook. Each answer is updated based on the current academic session as per the latest NCERT syllabus.
Detailed Explanations for Chapter 2 Is Matter Around Us Pure
Our expert teachers have provided step-by-step explanations for all the difficult questions in the Class 9 Science chapter. Along with the final answers, we have also explained the concept behind it to help you build stronger understanding of each topic. This will be really helpful for Class 9 students who want to understand both theoretical and practical questions. By studying these NCERT Questions and Answers your basic concepts will improve a lot.
Benefits of using Science Class 9 Solved Papers
Using our Science solutions regularly students will be able to improve their logical thinking and problem-solving speed. These Class 9 solutions are a guide for self-study and homework assistance. Along with the chapter-wise solutions, you should also refer to our Revision Notes and Sample Papers for Chapter 2 Is Matter Around Us Pure to get a complete preparation experience.
The complete and updated is available for free on StudiesToday.com. These solutions for Class 9 Science are as per latest NCERT curriculum.
Yes, our experts have revised the as per 2026 exam pattern. All textbook exercises have been solved and have added explanation about how the Science concepts are applied in case-study and assertion-reasoning questions.
Toppers recommend using NCERT language because NCERT marking schemes are strictly based on textbook definitions. Our will help students to get full marks in the theory paper.
Yes, we provide bilingual support for Class 9 Science. You can access in both English and Hindi medium.
Yes, you can download the entire in printable PDF format for offline study on any device.

