Practice CBSE Class 11 Chemistry Organic Chemistry Some Basic Principles and Techniques MCQs Set C provided below. The MCQ Questions for Class 11 Chapter 8 Organic Chemistry Some Basic Principles and Techniques Chemistry with answers and follow the latest CBSE/ NCERT and KVS patterns. Refer to more Chapter-wise MCQs for CBSE Class 11 Chemistry and also download more latest study material for all subjects
MCQ for Class 11 Chemistry Chapter 8 Organic Chemistry Some Basic Principles and Techniques
Class 11 Chemistry students should review the 50 questions and answers to strengthen understanding of core concepts in Chapter 8 Organic Chemistry Some Basic Principles and Techniques
Chapter 8 Organic Chemistry Some Basic Principles and Techniques MCQ Questions Class 11 Chemistry with Answers
Question: Methoxyethane and propanol are the examples of isomerism of the type
a) structural
b) position
c) functional
d) tautomerism
Answer: c
Question: Which of the following Fischer's projection fommla is identical to D-glyceraldehyde?
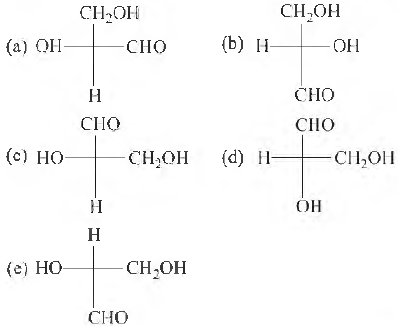
Answer: b
Question: Metamers of ethyl propionate are
a) C4H9COOH and HCOOC4H9
b) C4H9COOH and CH3COOC3H7
c) CH3COOCH3 and CH3COOC3H7
d) CH3COOC3H7 and C3H7COOCH3
Answer: d
Question: n-pentane, iso-pentane and neo-pentane are examples for isomers of the type
a) geometrical
b) optical
c) chain
d) positional
Answer: c
Question: Different structures generated due to rotation about, C—C axis, of an organic molecule, are examples of
a) geometrical isomerism
b) conformational isomerism
c) optical isomerism
d) structural isomerism
Answer: b
Question: For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values? (Assume ideal behaviour)
a) Heat of vaporisation
b) Vapour pressure at the same temperature
c) Boiling points
d) Gaseous densities at the same temperature and pressure
Answer: d
Question: Maximun1 enol content is in
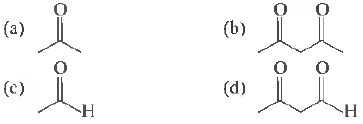
Answer: b
Question: Which organic structure among the following is not an isomer of the compound CH3–CO–CH2CH2CH2CH3 ?
a) CH3CH2OCH =CHCH2CH3
b) CH3CH = CHCH2CH2CHO
c) (CH3)2CH–CO–CH2CH3
d) CH3CH2COCH2CH2CH3
Answer: b
Question: Select the molecule which has only one π-bond
a) CH ≡ CH
b) CH2 = CHCHO
c) CH3CH = CH2
d) CH3CH=CHCOOH
Answer: c
Question: Increasing order of stability among the three main conformations, (i.e. Eclipse, Anti, Gauche) of 2-fluoroethanol is
a) Eclipse, Gauche, Anti
b) Gauche, Eclipse, Anti
c) Eclipse, Anti, Gauche
d) Anti, Gauche, Eclipse
Answer: c
Question: The compounds CH3CH == CHCH3 and CH3CH2CH == CH2
a) are tautomers
b) are position isomers
c) contain same number of sp3– sp3, sp3– sp2 and sp2– sp2 carbon-carbon bonds
d) exist together in dynamic equilibrium
Answer: b
Question: Heterolytic fission of C – Br bond results in the formation of
a) free radical
b) carbanion
c) carbocation
d) Both carbanion and carbocation
Answer: c
Question: Geometrical isomerism is possible in case of
a) pentene-2
b) propane
c) pentane
d) ethene
Answer: a
Question: In this reaction,
CH3CHO+HCN → CH3CH(OH)CN
→H·OH CH3CH(OH)COOH
an asymmetric centre is generated. The acid obtained would be
a) 50% D + 50% L-isomer
b) 20% D + 80% L-isomer
c) D-isomer
d) L-isomer
Answer: a
Question: The number of isomers of the compound with molecular fommla C2H2Br2 is
a) 4
b) 3
c) 5
d) 2
Answer: d
Question: C6H5C = N and C6H5N = C exhibit which type of isomerism?
a) Position
b) Functional
c) Metamerism
d) Dextro-isomerism
Answer: b
Question: How many isomers are possible for the alkane C4H10 ?
a) 3
b) 5
c) 2
d) 4
Answer: c
Question: The number of primary, secondary, tertiary and quaternary carbons in neopentane are respectively
a) 4, 3, 2 and 1
b) 5, 0, 0 and 1
c) 4, 0, 0 and 1
d) 4, 0, 1 and 1
Answer: c
Question: Which of the following compounds contains 1°, 2°, 3° as well as 4° carbon atoms ?
a) Neopentane
b) 2-methyl pentane
c) 2,3-dimethyl butane
d) 2,2,3-trimethyl pentane
Answer: d
Question: The number of secondary hydrogens in 2, 2-dimethylbutane is
a) 8
b) 6
c) 4
d) 2
Answer: d
Question: The organic reaction which proceed through heterolytic bond cleavage are called ________
a) ionic
b) polar
c) nonpolar
d) Both ionic and polar
Answer: d
Question: Which of the following statements is false for isopentane ?
a) It has three CH3 groups
b) It has one CH2 group
c) It has one CH group
d) It has a carbon which is not bonded to hydrogen
Answer: d
Question: The IUPAC name of neopentane is
a) 2, 2-dimethylpropane
b) 2-methylpropane
c) 2, 2-dimethylbutane
d) 2-methylbutane
Answer: a
Question: The IUPAC name of the compound CH3 — CH(CH3) — CO – CH3, is
a) 3-methyl 2-butanone
b) 2-methyl 3-butanone
c) isopropyl methyl ketone
d) methyl isopropyl ketone
Answer: a
Question: Total number of structural isomers possible for C3H6 are :
a) 2
b) 1
c) 4
d) 3
Answer: a
Question: Which of the following molecules is expected to rotate the plane of plane polarised light?
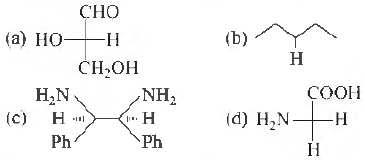
First organic compound to be synthesised was
a) methane
b) cane sugar
c) acetic acid
d) urea
Answer: a
Question: Which of the following statements is false for isopentane–
a) It has three CH3 groups
b) It has one CH2 group
c) It has one CH group
d) It has a carbon which is not bonded to hydrogen
Answer: d
Question: The IUPAC name of the compound CH3OCH2CH2CH2OCH2CH3 is
a) 3-ethoxy-1-methoxypropane
b) 1-ethoxy-3-methoxypropane
c) 2, 5-dioxyhexane
d) ethoxypropane oxymethane
Answer: a
Question: Which of the following compounds is optically active ?
a) (CH3 )2CHCH2OH
b) CH3CH2OH
c) CCI2F2
d) CH3CHOHC2H5
Answer: d
Question: C6H5C ≡ N and C6H5N ≡ C are which type of isomers?
a) Position
b) Functional
c) Tautomerism
d) Linkage
Answer: b
Question: A functional isomer of 1-butyne is
a) 2-butyne
b) 1-butene
c) 2-butene
d) 1, 3-butadiene
Answer: d
Question: In which of the following, functional group isomerism is not possible?
a) Alcohols
b) Aldehydes
c) Alkyl halides
d) Cyanides
Answer: c
Question: Which one of the following shows functional isomerism?
a) C2H4
b) C3H6
c) C2H5OH
d) CH2Cl2
Answer: c
Question: The least number of carbon atoms in alkane showing isomerism is
a) 3
b) 1
c) 2
d) 4
Answer: d
Question: The number of possible alkynes with molecular formula C5H8 is
a) 2
b) 3
c) 4
d) 5
Answer: b
Question: The functional group present in organic, acid is –
a) – OH
b) – CHO
c) –COOH
d) > C = O
Answer: c
Question: Amongst the following compounds, the optically active alkane having lowest molecular mass is
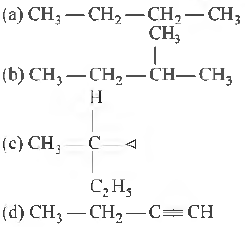
Answer: c
Question: How many asymmetric carbon atoms are present in
I. 1,2-dimethylcyclohexane
II. 3-methylcyclopentane and
III. 3-methylcyclohexene ?
a) Two, one, one
b) One, one, one
c) Two, none, two
d) Two, none, one
Answer: d
| CBSE Class 11 Chemistry Structure of Atom MCQs Set A |
| CBSE Class 11 Chemistry Structure of Atom MCQs Set B |
| CBSE Class 11 Chemistry Structure of Atom MCQs Set C |
| CBSE Class 11 Chemistry Classification of Elements and Periodicity in Properties MCQs Set A |
| CBSE Class 11 Chemistry Classification of Elements and Periodicity in Properties MCQs Set B |
| CBSE Class 11 Chemistry Periodic Classification of Elements MCQs |
| CBSE Class 11 Chemistry Redox Reactions MCQs Set A |
| CBSE Class 11 Chemistry Redox Reactions MCQs Set B |
| CBSE Class 11 Physics Kinetic Theory MCQs Set C |
Important Practice Resources for Class 11 Chemistry
MCQs for Chapter 8 Organic Chemistry Some Basic Principles and Techniques Chemistry Class 11
Students can use these MCQs for Chapter 8 Organic Chemistry Some Basic Principles and Techniques to quickly test their knowledge of the chapter. These multiple-choice questions have been designed as per the latest syllabus for Class 11 Chemistry released by CBSE. Our expert teachers suggest that you should practice daily and solving these objective questions of Chapter 8 Organic Chemistry Some Basic Principles and Techniques to understand the important concepts and better marks in your school tests.
Chapter 8 Organic Chemistry Some Basic Principles and Techniques NCERT Based Objective Questions
Our expert teachers have designed these Chemistry MCQs based on the official NCERT book for Class 11. We have identified all questions from the most important topics that are always asked in exams. After solving these, please compare your choices with our provided answers. For better understanding of Chapter 8 Organic Chemistry Some Basic Principles and Techniques, you should also refer to our NCERT solutions for Class 11 Chemistry created by our team.
Online Practice and Revision for Chapter 8 Organic Chemistry Some Basic Principles and Techniques Chemistry
To prepare for your exams you should also take the Class 11 Chemistry MCQ Test for this chapter on our website. This will help you improve your speed and accuracy and its also free for you. Regular revision of these Chemistry topics will make you an expert in all important chapters of your course.
You can get most exhaustive CBSE Class 11 Chemistry Organic Chemistry Some Basic Principles and Techniques MCQs Set C for free on StudiesToday.com. These MCQs for Class 11 Chemistry are updated for the 2025-26 academic session as per CBSE examination standards.
Yes, our CBSE Class 11 Chemistry Organic Chemistry Some Basic Principles and Techniques MCQs Set C include the latest type of questions, such as Assertion-Reasoning and Case-based MCQs. 50% of the CBSE paper is now competency-based.
By solving our CBSE Class 11 Chemistry Organic Chemistry Some Basic Principles and Techniques MCQs Set C, Class 11 students can improve their accuracy and speed which is important as objective questions provide a chance to secure 100% marks in the Chemistry.
Yes, Chemistry MCQs for Class 11 have answer key and brief explanations to help students understand logic behind the correct option as its important for 2026 competency-focused CBSE exams.
Yes, you can also access online interactive tests for CBSE Class 11 Chemistry Organic Chemistry Some Basic Principles and Techniques MCQs Set C on StudiesToday.com as they provide instant answers and score to help you track your progress in Chemistry.

