Revision Notes on Coordination Compounds
Ligands: an ion or molecule capable of donating a pair of electrons to the central atom via a donor atom.
• Unidentate ligands: Ligands with only one donor atom, e.g. NH3, Cl-, F- etc.
• Bidentate ligands: Ligands with two donor atoms, e.g. ethylenediamine, C2O42-(oxalate ion) etc.
• Tridentate ligands: Ligands which have three donor atoms per ligand, e.g. (dien) diethyl triamine.
• Hexadentate ligands: Ligands which have six donor atoms per ligand, e.g. EDTA.
Chelating Ligands:
• Multidentate ligand simultaneously coordinating to a metal ion through more than one site is called chelating ligand. Example: Ethylenediamine (NH2CH2CH2NH2)
• These ligands produce a ring like structure called chelate.
• Chelation increases the stability of complex
Werner’s Theory:
• Metals possess two types of valencies i.e. primary (ionizable) valency and secondary (nonionizable) valency.
• Secondary valency of a metal is equal to the number of ligands attached to it i.e. coordination number.
• Primary valencies are satisfied by negative ions, while secondary valencies may be satisfied by neutral, negative or positive ions.
• Secondary valencies have a fixed orientation around the metal in space.
[Co(NH3)6]Cl3
Primary Valencies = 3 Cl-
Secondary Valencies = 6 NH3
Coordination Sphere = [Co(NH3)6]3-
Nomenclature of Complexes:
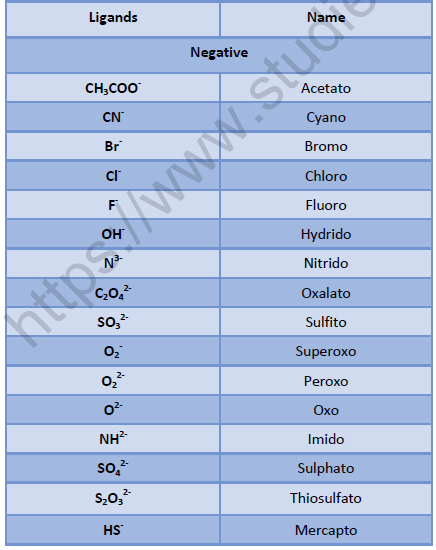
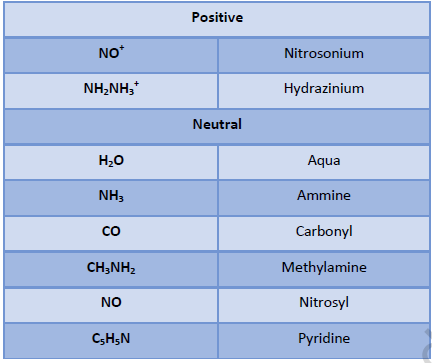
• Positive ion is named first followed by negative ion.
• Negative ligands are named by adding suffix - o.
• Positive ligands are named by adding prefix – ium.
• Neutral ligands are named as such without adding any suffix or prefix.
• Ligands are named in alphabetical order.
• Name of the ligands is written first followed by name of metal with its oxidation number mentioned in roman numbers in simple parenthesis.
• Number of the polysyllabic ligands i.e. ligands which have numbers in their name, is indicated by prefixes bis, tris etc, v Number and name of solvent of crystallization if any, present in the complex is written in the end of the name of complex.
• When both cation and anion are complex ions, the metal in negative complex is named by adding suffix-ate.
• In case of bridging ligands:
[Name of the groups to the left of bridging ligand (Oxidation state)] –μ – [Name of the groups to the right of bridging ligand (Oxidation state)] – [Name of negative ion]
Isomerism in coordination compounds
Structural Isomerism
• Ionization Isomerism: Exchange of ligands between coordinate sphere and ionization sphere
[Pt(NH3)4Cl2]Br2 & [Pt(NH3)4Br2]Cl2
• Hydrate Isomerism: Exchange of water molecules between coordinate sphere and ionization sphere
[Cr(NH3)3(H2O)3]Br3 & [Cr(NH32)3(H2O)2 Br]Br2 H2O
• Linkage Isomerism: Ambient legend binds from the different binding sites to the metal atom.
K2[Cu(CNS)4] & K2[Cu(SCN)4]
• Coordination Isomerism: Exchange of the metal atom between coordinate sphere and ionization sphere when both are complex ions.
[Cr(NH3)6][CoF6] & [Co(NH3)6][CrF6].
• Ligand Isomerism: Different isomers of the same ligands attached to the metal.
[Co(pn)2Br]Cl2 & [Co(tn)2Br]Cl2 Where,
pn = 1,2- Diaminopropane
tn = 1,3-Diaminopropane.
Stereoisomerism:
a. Geometrical Isomerism: When two similar ligands are on adjacent position the isomer is called cis isomer while hen they are on opposite positions, the isomer is called trans isomer.
b. Optical Isomerism: In order to show optical isomerism, the complex should form a non superimposible mirror image which rotates the place of polarized light in opposite direction.
Valence Bond Theory:
Hybridization:
Find out the hybridization of central metal ion using following steps:
• Write down the electronic configuration of metal atom.
• Find out oxidation state of metal atom.
• Write down the electronic configuration of metal ion.
• Write down the configuration of complex to find out hybridization.
• Strong field ligands cause the pairing of electrons.
Strong Field Ligands: CO, CN-, NO2-, en, py, NH3.
Weak Filed Ligands: H2O, OH-, F-, Cl-, Br-,I -
When the d orbital taking part in hybridization is inside the s and p orbital taking part in
hybridization with respect to the nucleus, it is called an inner orbital complex.
Example: d2sp3 hybridization of [Co(NH3)6]3+ involves 3d, 4s and 4p orbital, hence it is an inner orbital complex.
When the d orbital taking part in hybridization outside the s and p orbital taking part in hybridization with respect to the nucleus, it is called an outer orbital complex.
Example: sp3d2 hybridization of [CoF6]3- involves 4d, 4s and 4p orbital, hence it is an inner orbital complex.
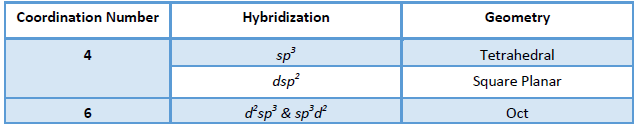
Geometry:
Magnetic Properties:
• Diamagnetic: All the electrons paired.
• Paramagnetic: Contains unpaired electrons.
Spin:
• Spin paired: All electrons paired.
• Spin free: Contains unpaired electrons.
Colour:
Compound must contain free electrons in order to show colour.
Crystal Field Theory:
Strong field ligand causes greater repulsion and thus results in the formation of low spin complexes by pairing of electrons.
• Weak field ligands result in the formation of high spin complexes
• Order of strength of ligands: CO > CN- > NO2 - > en > py = NH3 > H2O > OH- > F- > Cl- > Br- >I-
• Octahedral Complexes: eg orbital are of higher energy than t2g orbital.
• Tetrahedral Complexes: eg orbitals are of lower energy than t2g orbitals.
Δt = (4/9) Δo
Crystal Field Stabilization Energy:
Magnetic Properties: Complexes with unpaired electrons are paramagnetic while with no unpaired electron are diamagnetic.

