Modern Periodic Law:
• Properties of elements are the periodic function to their atomic numbers.
• The periodicity in properties is due to repetition of similar outer shell electronic configuration at a certain regular intervals.
• In modern periodic table is based on modern periodic law in which elements are arranged in increasing order of their atomic numbers.
• In the modern periodic table, the elements are arranged in rows and columns. These rows and columns are known as periods and groups respectively.
• The table consists of 7 periods and 18 groups
• Period indicates the value of ‘n’ (principal quantum number) for the outermost or valence shell.
• Same number of electrons is present in the outer orbitals (that is, similar valence shell electronic configuration
IUPAC Nomenclature for Elements with Atomic Number 100
Classification of Elements:
Periodicity in Atomic Properties:
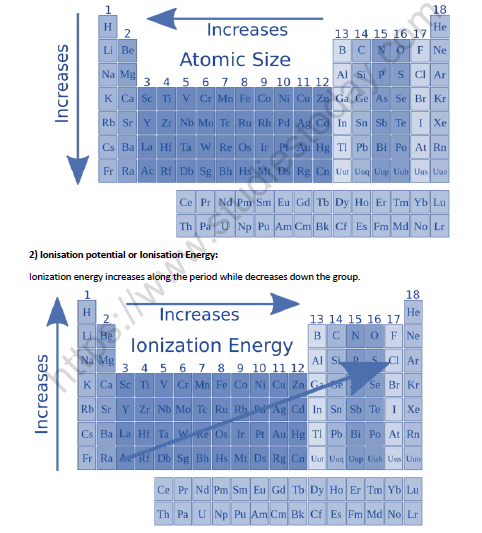
1. Atomic Radius:
• Within a given period atomic radius decreases from left to right. This is due to the effect of increase in nuclear charge while the electrons are being added to the same shell.
• Within a given group atomic radius increases down the group. This is due to the increase in number of shells.
• In the first transition series the atomic size slightly decreases from Sc to Mn because effect of effective nuclear charge is stronger than the shielding effect. The atomic size from Fe to Ni remains almost the same because both the effects balance each other.
• The atomic size from Cu to Zn slightly increases because shielding effect is more than effective nuclear charge due to d10 structure of Cu and Zn.
• Inner transition elements – As we move along the lanthanide series, there is a decrease in atomic as well as ionic radius. The decrease in size is regular in ions but not so regular in atoms. This is called lanthanide contraction.
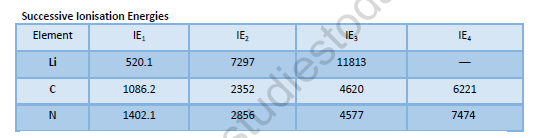
Factors which influence I.E.
• Atomic size: the larger the size of the atom, the smaller the I.E. i.e., I.E. μ
• Effective nuclear charge: The greater the effective charge on the nucleus of an atom, the more difficult it would be to remove an electron from the atom because electrostatic force of attraction between the nucleus and the outermost electron increases. So greater energy will be required to remove the electron.
• Penetration effect of orbitals: The order of energy required to remove electron from s,p,d-and¦- orbitals of a shell is s>p>d>¦.
• Shielding or screening effect: Screening effect results in decrease of force of attraction between the nucleus and the outermost electron and lesser energy is required to separate the electron. Thus the value of I.P. decreases.
• Stability of half-filled and fully-filled orbitals: According to Hund's rule the stability of half filled or completely filled degenerate orbitals is comparatively high. So comparatively more energy is required to separate the electron from such atoms.
3) Electron Affinity:
Electron affinity increases along the period while decreases down the group.
Factors affecting the magnitude of electron affinity
• Atomic size – In general electron affinity value decreases with the increasing atomic radius because electrostatic force of attraction decreases between the electron being added and the atomic nucleus due to increase of distance between them.
• Effective nuclear charge – Electron affinity value of the element increase as the effective nuclear charge on the atomic nucleus increases because electrostatic force of attraction between the electron being added and the nucleus increases. As the electrostatic force of attraction increases,amount of energy released is more.
• Screening or Shielding effect – Electron affinity value of the elements decreases with the increasing shielding or screening effect. The shielding effect between the outer electrons and the nucleus increases as the number of electrons increases in the inner shells.
Stability of half filled and completely filled orbitals – The stability of half filled and completely filled degenerate orbitals of a sub shell is comparatively more, so it is difficult to add electron in such orbitals and lesser energy is released on addition of electron hence the electron affinity value will decrease.
Please click the link below to download pdf file of NEET Chemistry Periodic Table Revision Notes

