Read and download the CBSE Class 11 Physics Thermodynamics Worksheet Set C in PDF format. We have provided exhaustive and printable Class 11 Physics worksheets for Chapter 11 Thermodynamics, designed by expert teachers. These resources align with the 2025-26 syllabus and examination patterns issued by NCERT, CBSE, and KVS, helping students master all important chapter topics.
Chapter-wise Worksheet for Class 11 Physics Chapter 11 Thermodynamics
Students of Class 11 should use this Physics practice paper to check their understanding of Chapter 11 Thermodynamics as it includes essential problems and detailed solutions. Regular self-testing with these will help you achieve higher marks in your school tests and final examinations.
Class 11 Physics Chapter 11 Thermodynamics Worksheet with Answers
Very Short Answer Type Questions :
Question. A gas does work during adiabatic expansion. What is the source of mechanical energy so produced ?
Answer : By first law of thermodynamics, ΔQ = ΔU + ΔW. But for an adiabatic process, ΔQ = 0, so ΔW = –ΔU. Thus the source of energy required for doing mechanical work during adiabatic expansion is the internal energy of the gas itself.
Question. A gas has two specific heats whereas a liquid and a solid have only one. Why?
Answer : When solids and liquids are heated, there is only a slight change in their volume and as such they possess only one specific heat, i.e., specific heat at constant volume. But in case of gases, pressure and volume both change and as such they possess two principal specific heats; one at constant pressure and one at constant volume.
Question. If no external energy is supplied to an expanding gas, will the gas do any work ? If yes, then what will be the source of energy ?
Answer : Yes, the gas will do work at the expense of its internal energy.
Question. What does the zeroth law of thermodynamics tell us about measuring the temperature of an object ?
Answer : Two objects in thermal equilibrium have the same temperature.
Question. A thermos bottle containing tea is vigorously shaken. What will be the effect on the temperature of tea?
Answer : Temperature of tea will rise.
Question. Is it possible to increase the temperature of a gas without adding heat to it? Explain.
Answer : Temperature of the gas can be increased by compressing the gas adiabatically. In this process, system requires no external heat supply.
According to first law of thermodynamics
ΔQ = ΔU + ΔW
For an adiabatic process, ΔQ = 0
- ΔU = –ΔW
When the gas is compressed, ΔW is negative hence ΔU is positive. So, the internal energy and therefore the temperature of the gas increases.
Question. First law of thermodynamics does not forbid flow of heat from lower temperature to higher temperature. Comment.
Answer : First law of thermodynamics simply tells about the conversion of mechanical energy into heat energy and viceversa. It does not put any condition as to why heat cannot flow from lower temperature to higher temperature.
Question. Which thermodynamic variable is defined by Zeroth law of thermodynamics?
Answer : Zeroth law of thermodynamics defines temperature.
Question. What is the nature of the internal energy of an ideal gas ?
Answer : Internal energy of an ideal gas is purely kinetic in nature.
Short Answer Type Questions :
Question. What is an isothermal process ? What are the essential conditions for an isothermal process to take place ?
Answer : A process in which temperature remains constant is called isothermal process. The essential conditions for an isothermal process to take place are :
(i) The walls of the container must be perfectly conducting to allow free exchange of heat between the gas and the surroundings.
(ii) The process of compression or expansion should be slow, so as to provide sufficient time for the exchange of heat.
Question. In changing the state of gas adiabatically from state of equilibrium A to state of equilibrium B. In this process 22.3 J work is done on the system. If the gas is taken from A to B via a process in which the net heat absorbed by the system is 9.35 cal, how much is the net work done by the system in later case ?
Answer : When the gas changes state under adiabatic condition (A → B)
ΔQ = 0 ⇒ ΔU = –ΔW = – 22.3 J
Since internal energy of the system is independent of path followed.
- In second case, ΔU = –22.3 J
ΔQ = 9.35 cal = (9.35 × 4.2) J = 39.27 J
ΔW = ΔQ – ΔU = 61.57 J
Question. An electric heater supplies heat to a system at a rate of 100 W. If system performs work at a rate of 75 joules per second. At what rate is the internal energy increasing?
Answer : Given that
Heat supplied, ΔQ = 100 W = 100 J s–1
Useful work done, ΔW = 75 J s–1
Change in internal energy, ΔU = ?
According to first law of thermodynamics, change in internal energy is given by
ΔU = ΔQ – ΔW
ΔU = 100 – 75 = 25 J s–1 = 25 W
Question. Two bodies at different temperatures T1 and T2, if brought in thermal contact do not necessarily settle to the mean temperature (T1 + T2)/2. Explain.
Answer : Let m1, m2 and c1, c2 be the masses and specific heats of the two bodies. Let T be their common temperature when these are brought in thermal contact. From the principle of calorimetry,
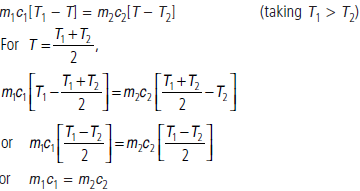
i.e., heat capacities of the two bodies should be equal.
Question. What do you mean by extensive and intensive state variables? Explain using suitable example.
Answer : Let us consider a system at equilibrium, and now it is divided into two equal parts. The variables that remain unchanged for each part are intensive. The variables whose value get halved in each part are extensive.
In other words, mass dependent variables are extensive and mass independent variables are intensive.
For e.g. : Internal energy (U), volume (V) and mass (M) are extensive variables.
Pressure (P), temperature (T) and density (r) are intensive variables.
Question. Two cylinders A and B of equal capacity are connected to each other via a stopcock. The cylinder A contains a gas at standard temperature and pressure, while the cylinder B is completely evacuated. The entire system is thermally insulated. The stopcock is suddenly opened.
Answer the following :
(a) What is the final pressure of the gas in A and B?
(b) What is the change in internal energy of the gas?
(c) What is the change in temperature of the gas?
Answer : (a) When the stopcock is suddenly opened, the volume available to the gas at 1 atm becomes twice the original volume and hence pressure becomes half the original volume (Boyle’s law). Hence the pressure of the gas in each of the cylinders A and B is 0.5 atm.
(b) As the system is thermally insulated, so ΔQ = 0. Also, the gas expands against zero pressure, so ΔW = 0. Hence by first law of thermodynamics, ΔU = 0 i.e., there is no change in the internal energy of the gas.
(c) As there is no change in the internal energy of the gas, so the temperature of the gas remains unchanged.
Question. A thermosflask contains coffee. It is vigorously shaken. Consider the coffee as the system. (a) Has any heat been added to it? (b) Has any work been done on it? (c) Has its internal energy changed? (d) Does its temperature rise?
Answer : (a) No. As the thermos flask is insulated, heat has not been added to the coffee (ΔQ = 0).
(b) Yes. Some work is done by the man in shaking the coffee against the forces of viscosity i.e., ΔW is negative.
(c) By first law of thermodynamics, ΔQ = ΔU + ΔW. As ΔQ = 0 and ΔW is negative, so ΔU is positive i.e., internal energy of the coffee increases.
(d) Because of the increase in internal energy of the coffee, the temperature of the coffee will also increase.
Question. A cylinder with a movable piston contains 3 moles of hydrogen at standard temperature and pressure. The walls of the cylinder are made of a heat insulator, and the piston is insulated by having a pile of sand on it. By what factor does the pressure of the gas increase if the gas is compressed to half its original volume?
Answer : As the gas is completely insulated, the process is adiabatic.
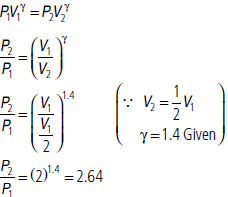
Question. The specific heat of hydrogen gas of constant pressure is CP = 3.4 × 103 cal/ kg °C and at constant volume is CV = 2.4 × 103 cal/kg °C. If one kilogram hydrogen gas is heated from 10°C to 20°C at constant pressure. What will be the external work done on the gas to maintain it at constant pressure?
Answer :
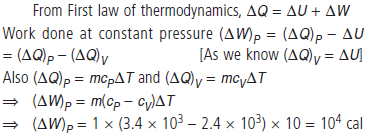
Question. Two samples of an ideal gas initially at the same temperature and pressure are allowed to expand from a volume V to 2V, one isothermally and other adiabatically. In which case, will
(a) the work done be more?
(b) the final pressure be more?
(c) the final temperature be more?
Justify your answers.
Answer : Figure shows the P-V diagrams for two gases expanded from volume V to 2V. As an adiabatic is steeper than an isotherm, so the adiabatic expansion curve AB lies below the isothermal expansion
curve AC.
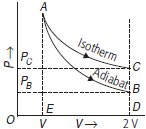
(a) Work done in adiabatic expansion = area ABDE
Work done in isothermal expansion = area ACDE
As area ACDE > area ABDE
So more work is done in the isothermal expansion.
(b) PB and PC are the final pressures for adiabatic and isothermal expansions respectively. Clearly, PC > PB. Hence the final pressure is greater for the isothermal expansion.
(c) In isothermal expansion, temperature remains constant T. In adiabatic expansion temperature decreases below T. So the final temperature is greater for the isothermal expansion.
Question. Define internal energy of a gas. Explain whether it is an extensive or intensive variable?
How internal energy of a gas can be changed ?
Answer : Internal energy : Internal energy of a system is the sum of kinetic energies and potential energies of the molecular constituents of the system. It does not include the overall kinetic energy of the system.
Internal energy is an extensive variable because it depends upon the mass and size (volume) of the system. Intensive variables like temperature, pressure does not depends upon the mass and size of the system.
Change in internal energy does not depend on the path of the process. It depends only on the initial and final states of the system i.e., ΔU = Uf – Ui.
Question. For one mole of gas, work done at constant pressure is W. What is the heat supplied at constant volume for the same rise in temperature of the gas ?
Answer : For the process at constant pressure
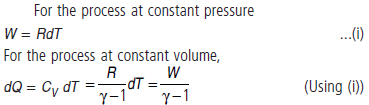
Question. An ideal gas has a molar heat capacity CV at constant volume. Find the molar heat capacity of this gas as a function of its volume V, if the gas undergoes the process T = T0eaV.
Answer :
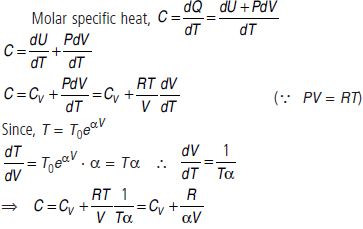
Question. If hot air rises up, why is it cooler at the top of mountain than near the sea level ? Explain.
Answer : As a rising hot air moves into regions of low pressure i.e., hill top, it expands. The air has to do work to expand, so its temperature must decrease. The only possible source of energy to do work for expansion is the thermal energy of the gas i.e., internal energy of gas. This is an adiabatic expansion as no thermal energy is transferred from surrounding. As a result, rising hot air become cooler at the top of mountain.
Question. When heat energy of 1500 J is supplied to a gas at constant pressure, 2.1 × 105 N m–2, there was an increase in its volume equal to 2.5 × 10–3 m3. What is the increase in its internal energy ?
Answer :
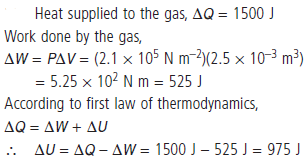
Question. Give difference between heat and work.
Answer : Heat : Heat is the energy transfer arising due to temperature difference between the system and the surroundings.
Work done : Work is energy transfer brought about by other means, such as moving the piston of a cylinder containing the gas, by raising or lowering some weight connected to it.
Question. Why is it impossible for a ship to use the internal energy of sea water to operate its engine ?
Answer : According to second law of thermodynamics, a working substance can do work only if its temperature is higher than that of its surroundings. Thus, if a ship is to use the internal energy of water to operate its engine, there should be a suitable sink to absorb the unused heat. The temperature of such a sink should be lower than that of sea water. Since there is no such sink available, the internal energy of sea water cannot be used to run a ship.
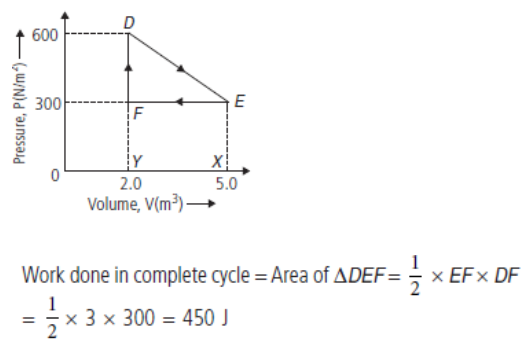
Question. Deduce the work done in the following complete cycle :
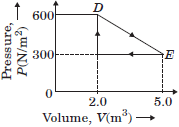
Answer :
Question. What do you understand by reversible and irreversible processes ? Give examples. What are the necessary conditions for a process to be reversible ?
Answer : Reversible Process : A thermodynamics process is reversible if the process can return back in such a way that both the system and surroundings return to their original states, with no other change anywhere else in the universe. It means both system and surroundings are returned to their initial states at the end of the reverse process.
Examples:
(i) The process of gradual compression and extension of an elastic spring is approximately reversible.
(ii) The process of electrolysis is reversible if the resistance offered by the electrolyte is negligibly small.
Irreversible process : An irreversible process is a thermodynamic process that departs from equilibrium. In this process, the system and surroundings do not return to their original condition once the process is initiated.
Examples :
(i) Diffusion of gases
(ii) Dissolution of salt in water
(iii) Rusting of iron
Necessary conditions for a reversible process :
(i) The process must be quasi-static. For this, the process must be carried out infinitesimally slowly so that the system remains in thermal and mechanical equilibrium with the surroundings throughout.
(ii) The dissipative force such as viscosity, friction, inelasticity, etc. should be absent.
Question. A system goes from P to Q by two different paths in the P-V diagram as shown in figure. Heat given to the system in path 1 is 1000 J. The work done by the system along path 1 is more than path 2 by 100 J. What is the heat exchanged by the system in path 2?
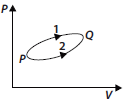
Answer : For path 2,
ΔQ = ΔU + ΔW …(i)
For path 1,
1000 = ΔU ′ + (ΔW + 100)
⇒ ΔU + ΔW = 900 (- ΔU = ΔU ′)
⇒ ΔQ = 900 J [Using eqn (i)]
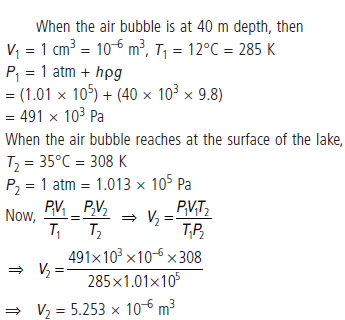
Question. An air bubble of volume 1.0 cm3 rises from the bottom of a lake 40 m deep at a temperature of 12°C. To what volume does it grow when it reaches the surface which is at a temperature of 35°C ?
Answer :
Long Answer Type Questions :
Question. A mixture of 1.78 kg of water and 262 g of ice at 0°C is, in a reversible process, brought to a final equilibrium state where the water / ice ratio, by mass is 1 : 1 at 0°C.
(a) Calculate the entropy change of the system during this process.
(b) The system is then returned to the first equilibrium state, but in an irreversible way (by using a Bunsen burner, for instance). Calculate the entropy change of the system during this process.
(c) Show that your answer is consistent with the second law of thermodynamics.
Answer : (a) Mass of water = 1.78 kg
Mass of ice = 262 g = 0.262 kg
So the total mass of ice and water mixture will be,
Mass of ice-water mixture
= (Mass of water) + (Mass of ice)
= 1.78 kg + 0.262 kg = 2.04 kg
If eventually the ice and water have the same mass, then the final state will have 1.02 kg (2.04 kg/2) of each. Thus the mass of the water that changed into ice m will be the difference of mass of water mw and mass of final state ms.
So, m = mw – ms
To obtain mass of water that changed into ice m, substitute 1.78 kg for mass of water mw and 1.02 kg for mass of final state ms in the equation m = mw – ms
= 1.78 kg – 1.02 kg = 0.76 kg
The change of water at 0°C to ice at 0°C is isothermal.
To obtain the change is entropy DS of the system during this process, substitute 0.76 kg for mass m, 333 × 103 J/kg for heat of fusion of water L and 273 K for T in the equation
ΔS = –mL/T
= –(0.76 kg) (333 × 103 J/kg)/(273 K) = –927 J/K
From the above ,we conclude that, the change in entropy ΔS of the system during this process will be –927 J/K.
(b) Now the system is returned to the first equilibrium state, but in an irreversible way. Thus the change in entropy ΔS of the system during this process is equal to the negative of previous case.
So, ΔS = –(– 927 J/K) = 927 J/K
From the above observation we conclude that, the change is entropy ΔS of the system would be 927 J/K.
(c) In accordance to second law of thermo-dynamics, entropy change ΔS is always zero. The total change in entropy will be,
ΔS = (–927 J/K) + (927 J/K) = 0
From the above observation we conclude that, our answer is consistent with the second law of thermodynamics.
Question. When a system is taken from state A to state B along the path ACB, 80 kcal of heat flows into the system and 30 kcal of work is done.
(a) How much heat flows into the system along path ADB if the work done is 10 kcal ?
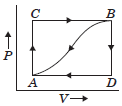
(b) When the system is returned from B to A along the curved path the work done is 20 kcal. Does the system absorb or librate heat?
(c) If UA = 0 and UD = 40 kcal, find the heat absorbed in the process AD.
Answer :

Question. (a) State first law of thermodynamics. Establish the relation between two principle molar specific heats of a gas on the basis of this law.
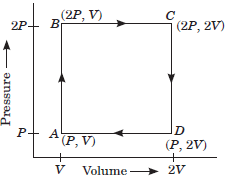
(b) An ideal gas is taken around the cycle ABCDA as shown in the P-V diagram. What is the work done during the cycle.
Answer : (a) The first law of thermodynamics is the general law of conservation of energy applied to any system in which energy transfer from or to the surroundings (through heat and work) is taken into account. It states that
ΔQ = ΔU + ΔW
where ΔQ is the heat supplied to the system, ΔW is the work done by the system and ΔU is the change in internal energy of the system.
Consider one mole of an ideal gas. Heat the gas to raise its temperature by ΔT. According to the first law of thermodynamics, the heat supplied ΔQ is used partly to increase the internal energy and partly expansion. That is, ΔQ = ΔU + PΔV
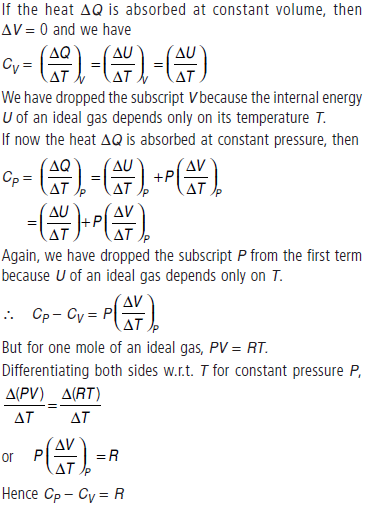
This is the required relation between CP and CV . It is also known as Mayer’s formula.
(b) Work done in the cyclic process ABCDA
= Area of the loop ABCD
= (2P – P) × (2V – V) = PV
Question. For the case of an ideal gas, find the equation of the process (in the variables T, V) in which the molar heat capacity varies as :
(a) C = CV + aT
(b) C = CV + bV
(c) C = CV + aP
where α, β, a are constants.
Answer :
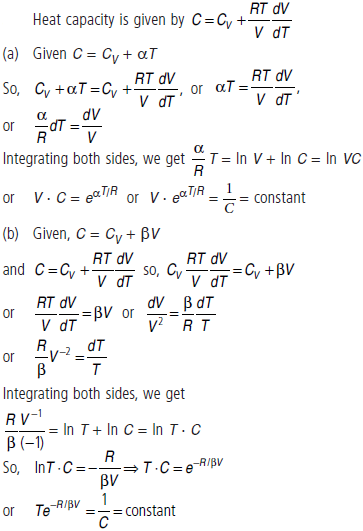
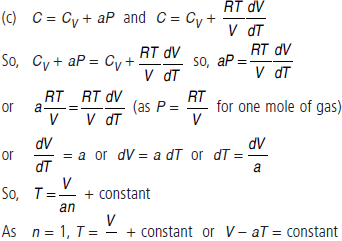
Question. Consider one mole of perfect gas in a cylinder of unit cross section with a piston attached as shown in figure. A spring (spring constant k) is attached (unstretched length L) to the piston and to the bottom of the cylinder. Initially the spring is unstretched and the gas is in equilibrium. A certain amount of heat Q is supplied to the gas causing an increase of volume from Vo to V
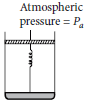
(a) What is the initial pressure of the system?
(b) What is the final pressure of the system?
(c) Using the first law of thermodynamics, write down a relation between Q, Pa, V, Vo and k.
Answer : Given, cross-sectional area of the piston
A = 1 m2
Heat supplied = Q.
Initial Volume = V0, Final volume = V
Atmospheric pressure pi = Pa
(a) Since system is in equilibrium so, initial pressure of the system, pi = Pa.
(b) When the gas expands from V0 to V after heat Q is supplied.
Change in volume of the gas = V – V0
![]()
Force applied by the spring on the piston
F = kx = k(V – V0)
So, pressure exerted by the extended spring on the piston of unit cross-section
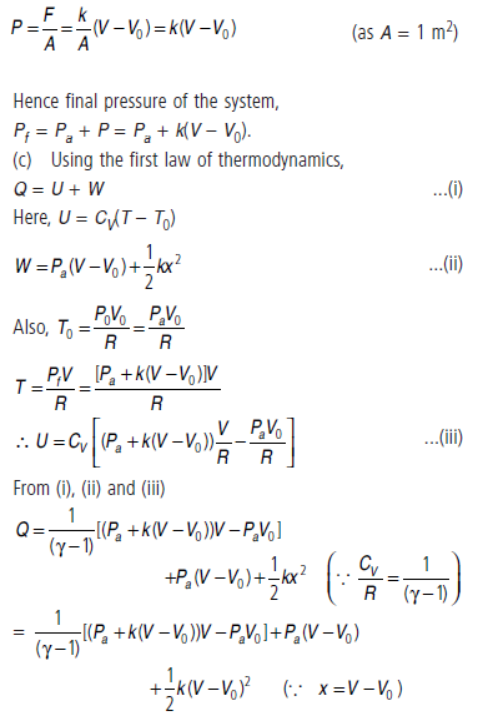
| CBSE Class 11 Physics Unit And Measurement Worksheet |
| CBSE Class 11 Physics Motion In Straight Line Worksheet |
| CBSE Class 11 Physics Motion In A Plane Worksheet |
| CBSE Class 11 Physics Laws Of Motion Worksheet |
| CBSE Class 11 Physics Work Energy And Power Worksheet |
| CBSE Class 11 Physics Systems Of Particles And Rotational Motion Worksheet |
| CBSE Class 11 Physics Gravitation Worksheet |
| CBSE Class 11 Physics Mechanical Properties Of Solids Worksheet Set A |
| CBSE Class 11 Physics Mechanical Properties Of Solids Worksheet Set B |
| CBSE Class 11 Physics Thermal Properties Of Fluids Worksheet |
| CBSE Class 11 Physics Thermal Properties Of Matter Worksheet Set A |
| CBSE Class 11 Physics Thermodynamics Worksheet Set A |
| CBSE Class 11 Physics Thermodynamics Worksheet Set B |
| CBSE Class 11 Physics Thermodynamics Worksheet Set C |
| CBSE Class 11 Physics Kinetic Theory Worksheet Set A |
| CBSE Class 11 Physics Kinetic Theory Worksheet Set B |
| CBSE Class 11 Physics Oscillations Worksheet Set A |
| CBSE Class 11 Physics Oscillations Worksheet Set B |
| CBSE Class 11 Physics Waves Worksheet Set A |
| CBSE Class 11 Physics Waves Worksheet Set B |
| CBSE Class 11 Physics Kinematics Worksheet |
| CBSE Class 11 Physics Rotational Motion Worksheet |
| CBSE Class 11 Physics System Of Particle And Rigid Body Worksheet |
| CBSE Class 11 Physics Physical World And Measurement Worksheet |
Important Practice Resources for Class 11 Physics
CBSE Physics Class 11 Chapter 11 Thermodynamics Worksheet
Students can use the practice questions and answers provided above for Chapter 11 Thermodynamics to prepare for their upcoming school tests. This resource is designed by expert teachers as per the latest 2026 syllabus released by CBSE for Class 11. We suggest that Class 11 students solve these questions daily for a strong foundation in Physics.
Chapter 11 Thermodynamics Solutions & NCERT Alignment
Our expert teachers have referred to the latest NCERT book for Class 11 Physics to create these exercises. After solving the questions you should compare your answers with our detailed solutions as they have been designed by expert teachers. You will understand the correct way to write answers for the CBSE exams. You can also see above MCQ questions for Physics to cover every important topic in the chapter.
Class 11 Exam Preparation Strategy
Regular practice of this Class 11 Physics study material helps you to be familiar with the most regularly asked exam topics. If you find any topic in Chapter 11 Thermodynamics difficult then you can refer to our NCERT solutions for Class 11 Physics. All revision sheets and printable assignments on studiestoday.com are free and updated to help students get better scores in their school examinations.
You can download the latest chapter-wise printable worksheets for Class 11 Physics Chapter Chapter 11 Thermodynamics for free from StudiesToday.com. These have been made as per the latest CBSE curriculum for this academic year.
Yes, Class 11 Physics worksheets for Chapter Chapter 11 Thermodynamics focus on activity-based learning and also competency-style questions. This helps students to apply theoretical knowledge to practical scenarios.
Yes, we have provided solved worksheets for Class 11 Physics Chapter Chapter 11 Thermodynamics to help students verify their answers instantly.
Yes, our Class 11 Physics test sheets are mobile-friendly PDFs and can be printed by teachers for classroom.
For Chapter Chapter 11 Thermodynamics, regular practice with our worksheets will improve question-handling speed and help students understand all technical terms and diagrams.

