Practice NTSE Chemistry Chemical Equilibrium MCQs provided below. The MCQ Questions for NTSE Chemical Equilibrium Chemistry with answers and follow the latest NTSE/ NCERT and KVS patterns. Refer to more Chapter-wise MCQs for NTSE NTSE Chemistry and also download more latest study material for all subjects
MCQ for NTSE Chemistry Chemical Equilibrium
NTSE Chemistry students should review the 50 questions and answers to strengthen understanding of core concepts in Chemical Equilibrium
Chemical Equilibrium MCQ Questions NTSE Chemistry with Answers
Question: Rate of an endothermic chemical reaction increases on -
- a) Decreasing the concentration of reactant
- b) Decreasing the surface area
- c) Decreasing the temperature
- d) None of these
Answer: Decreasing the temperature
Question: The function of an enzyme is a reaction of the type,
![]()
- a) Equilibrium constant
- b) Rate of forward reaction
- c) Rate of reverse reaction
- d) Activation energy.
Answer: Rate of reverse reaction
Question: Catalysts are those substance which -
- a) Only increase the rate of reaction
- b) Only decrease the rate of reaction
- c) Either increase or decrease the rate of reaction
- d) None of the above
Answer: None of the above
Question: Which of the following will react fastest ?
- a) Aluminum foil + NaOH + heat
- b) Aluminum foil + NaOH
- c) Aluminum powder + NaOH
- d) All will react equally
Answer: All will react equally
Question: What is the value of equilibrium constant K for the reaction
- a) K = 10-14
- b) K = 1
- c) K = 1.8 × 10-16
- d) K = 0
Answer: K = 10-14
Question: According to the law of mass action, the rate of reaction is directly proportional to the -
- a) Equilibrium constant
- b) Molar concentration of reactant (s)
- c) Volume
- d) Nature of reactant(s).
Answer: Molar concentration of reactant (s)
Question: During the process of photosynthesis, the product is -
- a) Sunlight
- b) Chlorophyll
- c) Carbon dioxide
- d) Glucose
Answer: Glucose
Question: Which of the following statements is incorrect ?
- a) In a multi step reaction, the rate determining step is the slowest one
- b) The specific rate constant for a reaction is independent of reactant concentration
- c) The rate of catalyzed reaction is always dependent on the concentration of the catalyst.
- d) The rise in temperature changes the specific rate constant of a reaction.
Answer: The rate of catalyzed reaction is always dependent on the concentration of the catalyst.
Question: Which of the following reactions will produce CO2 gas at the fasters rate ?
- a) 10 g Na2 CO3 with 20 ml of 2 M H2SO4
- b) 5 g Na2CO3 with 10 ml of 1 m H2SO4
- c) 5 g Na2CO3 with 10 ml of 2 M H2SO4
- d) Both (B) & (C) have same rate of production of CO2
Answer: 10 g Na2 CO3 with 20 ml of 2 M H2SO4
Question: Which of the following does not alter the concentration of the reactants and products are equilibrium ?
- a) Temperature
- b) Concentration
- c) Pressure
- d) Catalyst
Answer: Catalyst
More Questions...................
Question: If kp < kc then -
- a) n = 0
- b) n = + ve
- c) n= - ve
- d) Cannot determine
Answer: n= - ve
Question: Which of the following statements is correct about the equilibrium constant ?
- a) Its value increases by increase in temperature
- b) Its value decreases by decrease in temperature
- c) Its value may increase or decrease with increase in temperature
- d) Its value is constant at all temperatures
Answer: Its value may increase or decrease with increase in temperature
Question: When pressure is applied to the equilibrium system, Which of the following phenomenon will occur ?
- a) More ice will be formed
- b) Water will be evaporated
- c) More water will be formed
- d) Equilibrium will not be disturbed
Answer: More water will be formed
Question: At a given temperature, if there is a shift in equilibrium. The value of equilibrium constant will :
- a) Increase
- b) Decrease
- c) Remain constant
- d) May increase or decrease
Answer: Remain constant
Question: The oxidation of SO2 b O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum if
- a) Temperature in increased and pressure is kept constant
- b) Temperature is reduced and pressure is increased
- c) Both temperature and pressure and increased
- d) Both temperature and pressure and decreased
Answer: Temperature is reduced and pressure is increased
Question: The rate of forward reaction is two times that of the reverse reaction at a given temperature and identical concentration, K equilibrium is
- a) 0.5
- b) 1.5
- c) 2.5
- d) 2.0
Answer: 2.0
Question: In which of the following equilibrium, change in the volume of the system does not alter the number of moles.
- a)
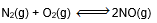
- b)
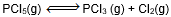
- c)
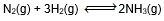
- d)
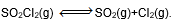
Answer:
![]()
Question: For the reaction -
![]()
the rate may be expressed as :
- a)
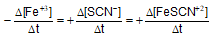
- b)
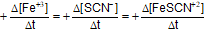
- c)
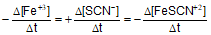
- d)
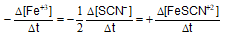
Answer:
![]()
Question: Consider the reaction -
![]()
In occurs in one step. The specific rate constants for the forward and the reverse reactions are 2.5 × 10-2 and 5 × 10-3 respectively. The equilibrium constant is :
- a) 1.25 × 102
- b) 5.0 × 10-6
- c) 2 × 105
- d) 0.05 + 102
Answer: 0.05 + 102
Question: In the reaction,
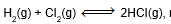 relation between KP and KC will be-
relation between KP and KC will be-
- a) KP = KC
- b) KP > KC
- c) KP < KC
- d) Cannot predict
Answer: KP = KC
Question: For a reversible reaction, -
![]()
expression for KC will be-
- a)
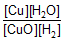
- b)
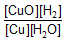
- c)
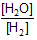
- d) None of these
Answer:
![]()
Question: For a reaction
The following data is given at any time at 298 K
[P] = 1 M, [Q] = 1 M, [R] = 1 M, [S] = 1 M.
the value of equilibrium constant Kc is 5. In which direction should the reaction proceed to attain equilibrium?
- a) Towards left hand side
- b) Towards right hand side
- c) The reaction will proceed at the same rate on both the sides
- d) The reaction will stop.
Answer: The reaction will proceed at the same rate on both the sides
Question: The following reaction was carried out in water
CI2 + 2I→I2 + 2CI-
The initial concentration of I- was 0.25 mol L-1 and the concentration after 10 minutes was 0.23 mol L-1 The rate of disappearance of I- and appearance of I2 is respectively -
- a) 0.002, 0.001 Mol L-1 min-1
- b) 0.001, 0.002 Mol L-1 min-1
- c) 0.002, 0.002 Mol L-1 min-1
- d) None of these
Answer: 0.001, 0.002 Mol L-1 min-1
Question: For following chemical reaction
![]()
KJ/mol. What would happen if the volume of the reaction vessel is doubled suddenly ?
- a) Unpredictable
- b) The equilibrium proceeds in the right direction.
- c) The equilibrium proceeds in the left direction
- d) Volume change doesn’t have any effect on equilibrium.
Answer: The equilibrium proceeds in the left direction
Question: The oxidation of SO2 b O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum if
- a) Temperature in increased and pressure is kept constant
- b) Temperature is reduced and pressure is increased
- c) Both temperature and pressure and increased
- d) Both temperature and pressure and decreased
Answer: Temperature is reduced and pressure is increased
Question: The reaction between Cr2O2 --- and HNO2 in an acidic medium is-
Cr2O2--+ 5H+ + 3HNO2 → 2Cr+++ + 3nO3- + 4H2O
The rate of disappearance of Cr2O7-- is found to be 2.4 × 10-4 Mol L-1 sec-1 during a measured time interval, the rate of disappearance of HNO2 will be-
- a) 4.8 × 10-4 Mol L-1 sec-1
- b) 3 × 2.4 × 10-4 Mol L-1 sec-1
- c) 7.2 × 10-4 Mol L-1 sec-1
- d) (B) & (C)
Answer: 4.8 × 10-4 Mol L-1 sec-1
Question: Consider the following equilibrium in a closed container :
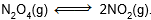 At a fixed temperature, the volume of the reaction container is halved. For this change which of the following statements holds true regarding the equilibrium constant (Kp) and degree of dissociation (α)
At a fixed temperature, the volume of the reaction container is halved. For this change which of the following statements holds true regarding the equilibrium constant (Kp) and degree of dissociation (α)
- a) Neither Kp and α nor changes
- b) Both Kp and α change
- c) Kp changes, but α does not change
- d) Kp does not change, but α changes
Answer: Kp does not change, but α changes
Question: At constant temperature, the equilibrium constant (Kp) for the decomposition reaction
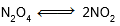 is expressed by Kp = 4x2P/(1 - x2) where P is pressure, x in extent of decomposition. Which of the following statement is true ?
is expressed by Kp = 4x2P/(1 - x2) where P is pressure, x in extent of decomposition. Which of the following statement is true ?
- a) Kp increases with increase of P
- b) Kp increases with increase of x
- c) Kp increases with decrease of x
- d) Kp remains constant with change is P or x
Answer: Kp remains constant with change is P or x
Question: For the reversible reaction
at 5000 C. The value of Kp is 1.44 × 10-5 when partial pressure is measured in atmospheres. The corresponding value of Kc with concentration is mol L-1 is-
- a) 1.44 × 10-5/(0.082 × 500) -5
- b) 1.44 × 10-5/(8.314 × 773) -2
- c) 1.44 × 10-5/(0.082 × 500)2
- d) 1.44 × 10-5/(0.082 × 773) -2
Answer: 1.44 × 10-5/(0.082 × 773) -2
Question: For the chemical reaction
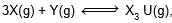 the amount of X3 Y at equilibrium is affected by
the amount of X3 Y at equilibrium is affected by
- a) Temperature and pressure
- b) Temperature only
- c) Pressure only
- d) Temperature, pressure and catalyst.
Answer: Temperature and pressure
Question: The oxidation of SO2 b O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum if
- a) Temperature in increased and pressure is kept constant
- b) Temperature is reduced and pressure is increased
- c) Both temperature and pressure and increased
- d) Both temperature and pressure and decreased
Answer: Temperature is reduced and pressure is increased
Question: The equilibrium constant for the following reaction will be- 3 A + 2B →C
- a)
- b)
- c)
- d)
Answer:
Question: The rate of forward reaction is two times that of the reverse reaction at a given temperature and identical concentration, K equilibrium is
- a) 0.5
- b) 1.5
- c) 2.5
- d) 2.0
Answer: 2.0
Question: When H2 and I2 are mixed and equilibrium is attained, then
- a) Amount of HI formed is equal to the amount of H2 dissociated
- b) HI dissociation stops
- c) The reaction stops completely
- d) None of these
Answer: None of these
Question: In which of the following equilibrium, change in the volume of the system does not alter the number of moles.
- a)
- b)
- c)
- d)
Answer:
Question: If K2 and K2 are the equilibrium constants of the equilibrium (a) and (b) respectively, what is the relationship between the two constants ?
- a)
- b)
K2 = (K1)2
- c)
- d)
K1 = K2.
Answer:
Question: At a given temperature, the equilibrium constant for the reactions
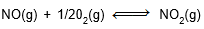 and
and 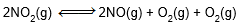 are K2 and K2 respectively. If K2 si 4 × 10-3 then K2 will be
are K2 and K2 respectively. If K2 si 4 × 10-3 then K2 will be
- a) 8 × 10-3
- b) 16 × 10-3
- c) 6.25 × 104
- d) 6.25 × 106
Answer: 6.25 × 104
Question: If Kp for a reaction
is 0.05 atm at 1000 K. Its Kc in terms of R will be
- a) 20000 R
- b) 0.02 R
- c) 5 × 10-5 R
- d)
Answer:
Question: Consider the reaction
in closed container at equilibrium. What would be the effect of additions of CaCO3 on the equilibrium concentration of CO2 ?
- a) Increases
- b) Decreases
- c) Data is not sufficient to predict it
- d) Remains unaffected
Answer: Remains unaffected
Question: For the redox reaction MnO4- + C2O42- + H+ → Mn2+ + CO2 + H2O the correct coefficients of the reactants for the balanced reaction are -
MnO4- C2O42- H+
- a) 2 5 16
- b) 16 5 2
- c) 5 16 2
- d) 2 16 5
Answer: 2 5 16
Question: In termite process, the reducing agent is -
- a) C
- b) Zn
- c) Na
- d) AI
Answer: AI
Question: Lead nitrate on heating gives lead oxide, nitrogen dioxide and oxygen. The reaction is known as -
- a) Decomposition
- b) Combustion
- c) Displacement
- d) Combination
Answer: Decomposition
Question: Rusting of iron is a chemical reaction. The reaction can be termed as -
- a) Displacement
- b) Oxidation
- c) Double decomposition
- d) Substitution
Answer: Oxidation
Question: The reaction, Na2SO4 + BaCI2 →BaSO4 + NaCI is -
- a) Decomposition
- b) Double displacement
- c) Substitution
- d) Combination
Answer: Double displacement
Question: When H2 and I2 are mixed and equilibrium is attained, then
- a) Amount of HI formed is equal to the amount of H2 dissociated
- b) HI dissociation stops
- c) The reaction stops completely
- d) None of these
Answer: None of these
| NTSE Chemistry Acids Bases and Salts MCQs |
| NTSE Chemistry Atomic Structure and Nuclear Chemistry MCQs Set A |
| NTSE Chemistry Atomic Structure and Nuclear Chemistry MCQs Set B |
| NTSE Chemistry Carbon Coal and Petroleum MCQs |
| NTSE Chemistry Chemical Bonding MCQs |
| NTSE Chemistry Chemical Equilibrium MCQs |
| NTSE Chemistry Metals and Non Metals MCQs Set A |
| NTSE Chemistry Metals and Non Metals MCQs Set B |
| NTSE Chemistry Mole Concept MCQs |
| NTSE Chemistry Nature Of Matter MCQs |
| NTSE Chemistry Periodic Table MCQs |
| NTSE Chemistry Pollution MCQs |
Important Practice Resources for NTSE SAT Chemistry s
MCQs for Chemical Equilibrium Chemistry NTSE
Students can use these MCQs for Chemical Equilibrium to quickly test their knowledge of the chapter. These multiple-choice questions have been designed as per the latest syllabus for NTSE Chemistry released by NTSE. Our expert teachers suggest that you should practice daily and solving these objective questions of Chemical Equilibrium to understand the important concepts and better marks in your school tests.
Chemical Equilibrium NCERT Based Objective Questions
Our expert teachers have designed these Chemistry MCQs based on the official NCERT book for NTSE. We have identified all questions from the most important topics that are always asked in exams. After solving these, please compare your choices with our provided answers. For better understanding of Chemical Equilibrium, you should also refer to our NCERT solutions for NTSE Chemistry created by our team.
Online Practice and Revision for Chemical Equilibrium Chemistry
To prepare for your exams you should also take the NTSE Chemistry MCQ Test for this chapter on our website. This will help you improve your speed and accuracy and its also free for you. Regular revision of these Chemistry topics will make you an expert in all important chapters of your course.
You can get most exhaustive NTSE Chemistry Chemical Equilibrium MCQs for free on StudiesToday.com. These MCQs for NTSE Chemistry are updated for the 2025-26 academic session as per NTSE examination standards.
Yes, our NTSE Chemistry Chemical Equilibrium MCQs include the latest type of questions, such as Assertion-Reasoning and Case-based MCQs. 50% of the NTSE paper is now competency-based.
By solving our NTSE Chemistry Chemical Equilibrium MCQs, NTSE students can improve their accuracy and speed which is important as objective questions provide a chance to secure 100% marks in the Chemistry.
Yes, Chemistry MCQs for NTSE have answer key and brief explanations to help students understand logic behind the correct option as its important for 2026 competency-focused NTSE exams.
Yes, you can also access online interactive tests for NTSE Chemistry Chemical Equilibrium MCQs on StudiesToday.com as they provide instant answers and score to help you track your progress in Chemistry.

