Practice NTSE Chemistry Atomic Structure and Nuclear Chemistry MCQs Set A provided below. The MCQ Questions for NTSE Atomic Structure and Nuclear Chemistry Chemistry with answers and follow the latest NTSE/ NCERT and KVS patterns. Refer to more Chapter-wise MCQs for NTSE NTSE Chemistry and also download more latest study material for all subjects
MCQ for NTSE Chemistry Atomic Structure and Nuclear Chemistry
NTSE Chemistry students should review the 50 questions and answers to strengthen understanding of core concepts in Atomic Structure and Nuclear Chemistry
Atomic Structure and Nuclear Chemistry MCQ Questions NTSE Chemistry with Answers
Question: The mass of proton is same as that of :
- a) Carbon atom
- b) An electron
- c) Hydrogen ion
- d) Oxygen atom
Answer: Hydrogen ion
Question: The ratio of the mass of the proton to the mass of the electron is nearly -
- a) 2000
- b) 4801
- c) 1840
- d) 8140
Answer: 1840
Question: Present unit of atomic mass is -
- a) Weight of proton
- b) Weight of one neutron
- c) Weight of one hydrogen atom
- d) 1/12 th of the weight of C-12 atom.
Answer: 1/12 th of the weight of C-12 atom.
Question: An element has atomic weight W and atomic number N. The number of protons in the nucleus of its atom is:
- a) W - N
- b) W
- c) N
- d) W + N
Answer: N
Question: The increasing order of the value of e/m (charge/mass) of an electron (e), proton (p), neutron (n) and alpha particle is -
- a) e,p,n,α
- b) n,p,e,α
- c) n,p,α,e
- d) n, α,p,e
Answer: n, α,p,e
Question:
- a) Isotopes
- b) Isobars
- c) Isomorphs
- d) Isotones
Answer: Isotones
Question: When alpha particles are sent through a gold foil most of them go straight through the foil. The reason is :
- a) Alpha particles are much heavier than the electrons
- b) Alpha particles are positively charged.
- c) Most part of the atom is empty.
- d) None of the above.
Answer: Most part of the atom is empty.
Question: Rutherford scattering experiment is related to the size of the -
- a) Nucleons
- b) Electron
- c) Atom
- d) Nucleus
Answer: Nucleus
Question: The maximum number of orbital in L - shell is -
- a) 1
- b) 4
- c) 3
- d) 6
Answer: 4
Question: Maximum number of orbital in N - shell is -
- a) 4
- b) 9
- c) 16
- d) 25
Answer: 16
More Questions.......................
Question: Which of the following has zero electron affinity ?
- a) Radon
- b) Nitrogen
- c) Oxygen
- d) Radium
Answer: Radon
Question: The isoelectronic species are -
- a)
- b)
- c)
- d)
Answer:
Question: An atom of an elements has 15 electrons in M shell and 2 electrons in N shell. Atomic number of the element will be:
- a) 2
- b) 15
- c) 17
- d) 27
Answer: 27
Question: If the electronic configuration of elements ‘A’ and ‘B’ are 1s2, 2s2, 2p6, 3s1 and 1s2, 2s2, 2p6, 3s2 3p4 respectively, then the formula of the compound formed by the combination of these elements will be-
- a) AB
- b) AB3
- c) AB2
- d) A2B
Answer: A2B
Question: Which of the following sets of orbital is arrange din the correct order of increasing energy ?
- a) 3d < 4s < 4p < 6s < 4d
- b) 2s < 3d < 4p < 4f < 1s
- c) 4s < 3d < 4p < 5s < 4d
- d) 1s < 2s < 2p < 4d < 3f
Answer: 4s < 3d < 4p < 5s < 4d
Question: Element ‘A’ with general outer shell configuration ns2 np5 usually exists as-
- a) A and forms A- ion.
- b) A and forms A+ ion.
- c) A2 and forms A- ion.
- d) A2 and forms A-2 ion.
Answer: A2 and forms A- ion.
Question: Atoms having different atomic number and same mass number are known as-
- a) Stones
- b) Isotopes
- c) Isobars
- d) Isomorphs
Answer: Isobars
Question: The particle with the least atomic mass is-
- a) proton
- b) neutron
- c) β particle
- d) α particle
Answer: β particle
Question: The time taken for 80g of uranium (t1/2= 4.5 × 109 years) to decay to 10 g would be -
- a) 4.5 × 109 years
- b) 9.0 × 109 years
- c) 13.5 × 109 years
- d) 18.0 × 109 years
Answer: 13.5 × 109 years
Question: If 92X238 liberates an α- particle then the number of neutrons present in daughter nuclei is -
- a) 144
- b) 146
- c) 92
- d) 143
Answer: 144
Question: In radioactive decay  the number of α and β particles emitted respectively are -
the number of α and β particles emitted respectively are -
- a) 0, 2
- b) 2, 0
- c) 1, 2
- d) 2, 1
Answer: 0, 2
Question: Consider the nuclear change, 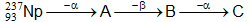 Which of the following statements is not correct ?
Which of the following statements is not correct ?
- a) age of deadwood.
- b) age of the sun.
- c) half-life of a radioactive element.
- d) amount of carbon in a substance
Answer: half-life of a radioactive element.
Question: In the study of plant respiration which of the following carbon is CO2 is used -
- a) C - 12
- b) C - 13
- c) C - 14
- d) C - 15
Answer: C - 12
Question: Which of the following statements is wrong ?
- a) P-33 is used in the treatment of Leukemia.
- b) I-131 is used in the treatment of Thyroid Gland disorder.
- c) Co-59 can’t be used for treatment of cancer.
- d) Excessive used of radioactive elements is responsible for cancerous growth
Answer: Excessive used of radioactive elements is responsible for cancerous growth
Question: The ratio of e/m for a cathode ray :
- a) varies with a gas in a discharge tube.
- b) is fixed.
- c) varies with different electrodes.
- d) is maximum if hydrogen is taken.
Answer: varies with a gas in a discharge tube.
Question: Atoms may be regarded as comprising of protons, neutrons and electrons. If the mass attributed by electrons was doubled and the attributed by neutrons was halved, the atomic mass of 12C would be -
- a) approximately the same.
- b) doubled.
- c) reduced approximately 25%.
- d) approximately halved.
Answer: doubled.
Question: The size of a nucleus is measured in-
- a) amu
- b) Angstrom
- c) cm
- d) Fermi
Answer: cm
Question: The highest value of e/m of anode rays has been observed when the discharge tube is filled with -
- a) Nitrogen
- b) Oxygen
- c) Hydrogen
- d) Helium
Answer: Helium
Question: The number of neutrons in dipositive zinc ion (Zn2+ with mass number 70) is-
- a) 34
- b) 36
- c) 38
- d) 40
Answer: 38
Question: Isotopes are identified by-
a) Positive ray analysis.
b) Aston’s mass spectrograph
c) Dempster’s mass spectrograph.
d) All the above
Answer: D
Question. Nucleons are:
a. Protons and electrons
b. Protons and neutrons
c. Electrons and neutrons
d. Electrons, protons and neutrons
Answer : B
Question. 6C14 is formed from 7N14 in the upper atmosphere by the action of the fundamental particle:
a. Positron
b. Neutron
c. Electron
d. Proton
Answer : B
Question. A particle having the same charge and 200 times greater mass than that of electron is:
a. Positron
b. Proton
c. Neutrino
d. Meson
Answer : D
Question. The positron is discovered by:
a. Pauling
b. Anderson
c. Yukawa
d. Segar
Answer : B
Question. Which of the following does not contain number of neutrons equal to that of 4018Ar ?
a. 4119K
b. 4321Sc
c. 4021Sc
d. 4220Ca
Answer : C
Question. α-particles can be detected using:
a. Thin aluminum sheet
b. Barium sulphate
c. Zinc sulphide screen
d. Gold foil
Answer : C
Question. Which is the correct statement?
a. Isotopes are always radioactive
b. β -rays are always negatively charged particles
c. α -rays are always negatively charged particles
d. γ -rays can be deflected in magnetic field
Answer : B
Question. Which statement is incorrect?
a. α -rays have more penetrating power than β -rays
b. α -rays have less penetrating power than γ -rays
c. β -rays have less penetrating power than γ -rays
d. β -rays have more penetrating power than α -rays
Answer : A
Question. The velocity of α -rays is approximately:
a. Equal to that of the velocity of light
b. 1/10 of the velocity of light
c. 10 times more than the velocity of light
d. Un-comparable to the velocity of light
Answer : B
Question. Radioactivity was discovered by:
a. Henry Becquerel
b. Rutherford
c. J. J. Thomson
d. Madam Curie
Answer : A
Question. α -rays have:
a. Positive charge
b. Negative charge
c. No charge
d. Sometimes positive charge and sometimes negative charge
Answer : A
Question. The half-life period of a radioactive substance is 8 years.
After 16 years, the mass of the substance will reduce from starting 16.0g to:
a. 8.0g
b. 6.0 g
c. 4.0 g
d. 2.0 g
Answer : C
Question. The atomic mass of an element is 12.00710 amu. If there are 6 neutrons in the nucleus of the atom of the element, the binding energy per nucleon of the nucleus will be:
a. 7.64 MeV
b. 76.4 MeV
c. 764 MeV
d. 0.764 MeV
Answer : A
Question. The 88Ra226 is:
a. n-mesons
b. u-mesons
c. Radioactive
d. Non-radioactive
Answer : C
Question. The charge on gamma rays is:
a. Zero
b. +1
c. –1
d. +2
Answer : A
Question. 1.0g of a radioactive isotope was found to reduce to 125 mg after 24 hours. The half-life of the isotope is:
a. 8 hours
b. 24 hours
c. 6 hours
d. 4 hours
Answer : A
Question. A freshly prepared radioactive source of half-life 2 hours emits radiations of intensity which is 64 times the permissible safe level. The minimum time after which it would be possible to work safely with this source is?
a. 6 hours
b. 12 hours
c. 24 hours
d. 128 hours
Answer : B
Question. Which of the following nuclides has the magic number of both protons and neutrons?
a. 50Sn115
b. 82Pb206
c. 82Pb208
d. 50Sn118
Answer : C
Question. Positron has nearly the same weight as that of:
a. α -particle
b. Proton
c. Neutron
d. Electron
Answer : D
Question. Hydrogen and deuterium differ in:
a. Reactivity with oxygen
b. Reactivity with chlorine
c. Melting point
d. Reducing action
Answer : C
Question. In the sequence of following nuclear reactions
The value of n will be:
a. 3
b. 4
c. 5
d. 6
Answer : B
Question.
In the above sequence of reaction, the elements which are isotopes of each other are?
a. X and W
b. Y and Z
c. X and Z
d. None of these
Answer : A
Question. The nucleus of an atom is made up of X protons and Y neutrons. For the most stable and abundant nuclei
a. X and Y are both even
b. X and Y are both odd
c. X is even and Y is odd
d. X is odd and Y is even
Answer : A
Question. How many neutrons are present in the nucleus of Ra ?
a. 88
b. 226
c. 140
d. 138
Answer : D
Question. What is the value of decay constant of a compound having half-life time T1/ 2 = 2.95 days?
a. 2.7 x 10-5 s−1
b. 2.7 x 106 s−1
c. 2.7 x 10-6 s−1
d. 3 x 105 s−1
Answer : C
Question. What is the half-life of a radioactive substance if 75% of a given amount of the substance disintegrates in 30 minutes?
a. 7.5 minutes
b. 25 minutes
c. 20 minutes
d. 15 minutes
Answer : D
Question. A radioactive isotope decays at such a rate that after 96 minutes only 1/8th of the original amount remains. The half-life of this nuclide in minutes is:
a. 12
b. 24
c. 32
d. 48
Answer : C
Question. The half-life of 92U238 is 4.5×109 years. After how many years, the amount of 92U238 will be reduced to half of its present amount?
a. 9.0 × 109 years
b. 13.5 × 109 years
c. 4.5 × 109 years
d. 4.5 × 104.5 years
Answer : C
Question. A radioactive isotope decays at such a rate that after 192 minutes only 1 /16 of the original amount remains. The half-life of the radioactive isotope is:
a. 32 min
b. 48 min
c. 12 min
d. 24 min
Answer : B
Question. Two Cu64 nuclei touch each other. The electrostatics repulsive energy of the system will be:
a. 0.788 MeV
b. 7.88 MeV
c. 126.15 MeV
d. 788 MeV
Answer : C
Question. When 92U235 undergoes fission. 0.1% of its original mass is changed into energy. How much energy is released if 1kg of 92U235 undergoes fission?
a. 9 ×1010 J
b. 9 ×1011 J
c. 9 ×1012 J
d. 9 ×1013 J
Answer : D
Question. A radioactive substance takes 20 min to decay 25%. How much time will be taken to decay 75%?
a. 96.4 min
b. 68 min
c. 964 min
d. 680 min
Answer : A
Question. A heavy nucleus at rest breaks into two fragments which fly off with velocities in the ratio 8 : 1. The ratio of radii of the fragments is?
a. 1 : 2
b. 1 : 4
c. 4 : 1
d. 2 : 1
Answer : A
Question. The ratio of radii of nuclei 2713Al and 12552Te is approximately:
a. 6 : 10
b. 13 : 52
c. 40 : 177
d. 14 : 7
Answer : A
Question. If Avogadro’s number is 6×1023 then the number of protons, neutrons and electrons in 14g of 6C14 are respectively:
a. 36 × 1023 , 48 × 1023 , 36 × 1023
b. 36 × 1023 , 36 × 1023 , 36 × 1021
c. 48 × 1023 , 36 × 1023 , 48 × 1021
d. 48 × 1023 , 48 × 1023 , 36 × 1021
Answer : A
Question. The binding energy per nucleon of O16 is 7.97 MeV and that of O17 is 7.75 MeV. The energy (in MeV) required removing a neutron from O17 is:
a. 3.52
b. 3.64
c. 4.23
d. 7.86
Answer : C
Question. 10 gm of a radioactive substance is reduced to 1.25 gm after 15 days. Its 1 kg mass will reduce (in how many days) to 500 gm in?
a. 500 days
b. 125 days
c. 25 days
d. 5 days
Answer : D
Question. A radioactive isotope having a half-life of 3 days was received after 12 days. It was found that there were 3 gm of the isotope in the container. The initial weight of the isotope when packed was:
a. 12 gm
b. 24 gm
c. 36 gm
d. 48 gm
Answer : D
Question. A radioactive isotope has a half-life of 10 days. If today 125 mg is left over, what was its original weight 40 days earlier?
a. 2g
b. 600 mg
c. 1 g
d. 1.5 g
Answer : A
Question. Half-life of a radioactive substance which disintegrates by 75 % in 60 minutes, will be?
a. 120 min
b. 30 min
c. 45 min
d. 20 min
Answer : B
Question. The decay constant of Ra226 is 1.37 x 10−11 sec . A sample of Ra226 having an activity of 1.5 mill curie will contain...... atoms:
a. 4.1×1018
b. 3.7 ×1017
c. 2.05×1015
d. 4.7×1010
Answer : A
Question. 8 gm 0f the radioactive isotope, cesium-137 were collected on February 1 and kept in a sealed tube. On July 1, it was found that only 0.25 gm of it remained. So the half-life period of the isotope is:
a. 37.5 days
b. 30 days
c. 25 days
d. 50 days
Answer : B
Question. A gamma ray photon creates an electron-positron pair. If the rest mass energy of an electron is 0.5 MeV and the total kinetic energy of the electron-positron pair is 0.78 MeV, then the energy of the gamma ray photon must be:
a. 0.78 MeV
b. 1.78 MeV
c. 1.28 MeV
d. 0.28 MeV
Answer : B
Question. Binding energy per nucleon vs mass number curve for nuclei is shown in the figure. W, X, Y and Z are four nuclei indicated on the curve. The process that would release energy is:
a. Y → 2Z
b. W → X + Z
c. W → 2Y
d. X → Y + Z
Answer : C
Question. A radioactive substance decays to 1/16th of its initial activity in 40 days. The half-life of the radioactive substance expressed in days is:
a. 2.5
b. 5
c. 10
d. 20
Answer : C
Question. A nucleus with mass number 220 initially at rest emits an α-particle. If the Q value of the reaction is 5.5 MeV.
Calculate the kinetic energy of the α-particle:
a. 4.4 MeV
b. 5.4 MeV
c. 5.6 MeV
d. 6.5 MeV
Answer : B
Question. Let p m be the mass of a proton, n m the mass of a neutron, M1 the mass of a 2010Ne nucleus and M2 the mass of a 4020Ca nucleus. Then:
a. M2 = 2M1
b. M2 > 2M1
c. M2 < 2M1
d. M1 < 10(mn + mp)
Answer : C, D
Question. A sample of radioactive element has a mass of 10 gm at an instant t = 0. The approximate mass of this element in the sample after two mean lives is:
a. 2.50 gm
b. 3.70 gm
c. 6.30 gm
d. 1.35 gm
Answer : D
Question. Elements having different nuclear charge but the same mass number are called:
a. Isotopes
b. Isobars
c. Isomers
d. Isotones
Answer : B
Question. Emission of β -particle by an atom of an element results in the formation of its:
a. Isotope
b. Isomer
c. Isomorph
d. Isobar
Answer : D
Question. The half-life of 215At is 100 μs. The time taken for the radioactivity of a sample of 215At to decay to 1/16th of its initial value is:
a. 400 μs
b. 6.3 μs
c. 40 μs
d. 300 μs
Answer : A
Question. The mean lives of a radioactive substance for α and β emissions are 1620 years and 405 years respectively. After how much time will the activity be reduced to one fourth?
a. 405 year
b. 1620 year
c. 449 year
d. None of these
Answer : C
Question. Tritium is an isotope of:
a. Hydrogen
b. Titanium
c. Tantalum
d. Tellurium
Answer : A
Question. 1 g of hydrogen is converted into 0.993 g of helium in a thermonuclear reaction. The energy released is:
a. 63 ×107 J
b. 63 ×1010 J
c. 63 ×1014 J
d. 63 ×1020 J
Answer : B
Question. The binding energy per nucleon of deuteron (21H) and helium nucleus (42He) is 1.1 MeV and 7MeV respectively. If two deuteron nuclei react to form a single helium nucleus, then the energy released is:
a. 13.9 MeV
b. 26.9 MeV
c. 23.6 MeV
d. 19.2 MeV
Answer : C
Question. The masses of neutron and proton are 1.0087 amu and 1.0073 amu respectively. If the neutrons and protons combine to form a helium nucleus (alpha particles) of mass 4.0015 amu. The binding energy of the helium nucleus will be: [1 amu= 931 MeV]
a. 28.4 MeV
b. 20.8 MeV
c. 27.3 MeV
d. 14.2 MeV
Answer : A
Question. A atomic power reactor furnace can deliver 300 MW. The energy released due to fission of each of uranium atom U238 is 170 MeV. The number of uranium atoms fissioned per hour will be:
a. 5 × 1015
b. 10 × 1020
c. 40 × 1021
d. 30 × 1025
Answer : C
Question. In the nuclear fusion reaction 21H + 31H → He + n, given that the repulsive potential energy between the two nuclei is 14 7.7 10 J, − × − the temperature at which the gases must be heated to initiate the reaction is nearly?
[Boltzmann’s constant k = 1.38 x 10−23 J/K]
a. 109 K
b. 107 K
c. 105 K
d. 103 K
Answer : A
Question. Which of the following is an isobaric pair?
a. 6C13 , 7N13
b. 6C13 , 7N14
c. 7N14 , 8O15
d. 7N13 , 8O15
Answer : A
Question. Which one of the following pairs represents isobars?
a. 32He and 42He
b. 2412Mg and 2512Mg
c. 4019K and 4020Ca
d. 3919K and 4019K
Answer : C
| NTSE Chemistry Acids Bases and Salts MCQs |
| NTSE Chemistry Atomic Structure and Nuclear Chemistry MCQs Set A |
| NTSE Chemistry Atomic Structure and Nuclear Chemistry MCQs Set B |
| NTSE Chemistry Carbon Coal and Petroleum MCQs |
| NTSE Chemistry Chemical Bonding MCQs |
| NTSE Chemistry Chemical Equilibrium MCQs |
| NTSE Chemistry Metals and Non Metals MCQs Set A |
| NTSE Chemistry Metals and Non Metals MCQs Set B |
| NTSE Chemistry Mole Concept MCQs |
| NTSE Chemistry Nature Of Matter MCQs |
| NTSE Chemistry Periodic Table MCQs |
| NTSE Chemistry Pollution MCQs |
Important Practice Resources for NTSE SAT Chemistry s
MCQs for Atomic Structure and Nuclear Chemistry Chemistry NTSE
Students can use these MCQs for Atomic Structure and Nuclear Chemistry to quickly test their knowledge of the chapter. These multiple-choice questions have been designed as per the latest syllabus for NTSE Chemistry released by NTSE. Our expert teachers suggest that you should practice daily and solving these objective questions of Atomic Structure and Nuclear Chemistry to understand the important concepts and better marks in your school tests.
Atomic Structure and Nuclear Chemistry NCERT Based Objective Questions
Our expert teachers have designed these Chemistry MCQs based on the official NCERT book for NTSE. We have identified all questions from the most important topics that are always asked in exams. After solving these, please compare your choices with our provided answers. For better understanding of Atomic Structure and Nuclear Chemistry, you should also refer to our NCERT solutions for NTSE Chemistry created by our team.
Online Practice and Revision for Atomic Structure and Nuclear Chemistry Chemistry
To prepare for your exams you should also take the NTSE Chemistry MCQ Test for this chapter on our website. This will help you improve your speed and accuracy and its also free for you. Regular revision of these Chemistry topics will make you an expert in all important chapters of your course.
You can get most exhaustive NTSE Chemistry Atomic Structure and Nuclear Chemistry MCQs Set A for free on StudiesToday.com. These MCQs for NTSE Chemistry are updated for the 2025-26 academic session as per NTSE examination standards.
Yes, our NTSE Chemistry Atomic Structure and Nuclear Chemistry MCQs Set A include the latest type of questions, such as Assertion-Reasoning and Case-based MCQs. 50% of the NTSE paper is now competency-based.
By solving our NTSE Chemistry Atomic Structure and Nuclear Chemistry MCQs Set A, NTSE students can improve their accuracy and speed which is important as objective questions provide a chance to secure 100% marks in the Chemistry.
Yes, Chemistry MCQs for NTSE have answer key and brief explanations to help students understand logic behind the correct option as its important for 2026 competency-focused NTSE exams.
Yes, you can also access online interactive tests for NTSE Chemistry Atomic Structure and Nuclear Chemistry MCQs Set A on StudiesToday.com as they provide instant answers and score to help you track your progress in Chemistry.

