1 . INTRODUCTION :
Matter can exist in three physical states namely ; solid, liquid and gas. Matter consists of tiny particles (atoms, ions or molecules). If the particles are very far off from one another, they behave like gases; nearer, they behave like liquids, and nearest, like solids. The three states of matter are thus known as the three
states of aggregation from Latin word meaning "Flacking together".
The fundamental difference between the three states of aggregation lies essentially in the difference of the relative amounts of energy possessed by the particles in the three states. The relative energies in the different states of matter are governed by two universal opposing tendencies associated with the particles :
(i) They have tendency of mutual attraction.
(ii) They have tendency of escape from one another which is known as escaping tendency.
Whether a given system would exist as a solid, liquid or gas depends upon the relative strengths of these opposing tendencies. If the escaping tendency is greater than the attraction between them, the molecules will be carried far from each other to distances which are large as compared with their diameters, the system will exists in gaseous state. But in the liquid state the molecular attraction exceeds the escaping tendency and in the solid state the forces of attraction are so much greater than those of escaping tendency that each particle is bound into a definite place in a rigid position by the mutual attraction of molecules. In other words, in the solid state, the system possesses the amount of energy of motion i.e. kinetic energy.
2 . THE SOLID STATE :
The solid are characterised by incompressibility, rigidity and mechanical strength. The molecules, atoms or ions in solids are closely packed i.e. they are held together by strong forces and can not move about at random. Thus solids have definite volume, shape, slow diffusion, low vapour pressure and possesses the unique property of being rigid. Such solids are known as true solids e.g. NaCl, KCl, Sugar, Ag, Cu etc.
On the other hand the solid which loses shapes on long standing, flows under its own weight and easily distorted by even mild distortion forces are called pseudo solids e.g. glass, pitch etc.
Some solids such as NaCl, Sugar, Sulphur etc. have properties not only of rigidity and incompressibility but also of having typical geometrical forms. These solids are called as crystalline solids. In such solids there is definite arrangements of particles (atoms, ions or molecules) throughout the entire three dimensional network of a cr ystal. This is named as long-range order. This three dimensional arrangement is called cr ystal lattice or space lattice. Other solids such as glass, rubber, plastics etc. have rigidity and incompressibility to a certain extent but they do not have definite geometrical forms or do not have long range order are known as amorphous solids.
3 . DIFFERENCES BET WEEN CRYSTALLINE AND AMORPHOUS SOLIDS :
( i ) Characteristic Geometry : In the crystalline solids the particles (atoms, ions, or molecules) are definitely and orderly arranged thus these have characteristic geometry while amorphous solids do not have characteristic geometry.
( ii ) Melting Points : A crystalling solids has a sharp melting point i.e. it changes into liquid state at a definite temperature. On the contrary an amorphous solid does not has a sharp melting point. For example, when glass is heated, it softens and then starts flowing without undergoing any abrupt or sharp change from solid to liquid state. Therefore, amorphous solids are regarded as "liquids at all temperatures".
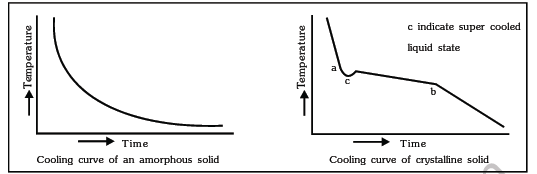
( iii ) Cooling curve : Amorphous solids show smooth cooling curve while crystalline solids show two breaks in cooling curve. In the case of crystalline solids two breaks points 'a' and 'b' are appear. These points indicate the beginning and the end of the process of crystallization. In this time interval temperature remains constant. This is due to the fact that during crystallisation process energy is liberated which compensates for the loss of heat thus the temperature remains constant.
( i v ) Isotropy and Anisotropy : Amorphous solids differ from crystalline solids and resemble liquids in many respects. The properties of amorphous solids, such as electrical conductivity, thermal conductivity,mechanical strength, refractive index, coefficient of thermal expansion etc. are same in all directions.Such solids are known as isotropic. Gases and liquids are also isotropic.
On the other hand crystalline solids show these physical properties different in diferent directions.Therefore crystalline solids are called anisotropic. The anisotropy itself is a strong evidence for the existence of orderly molecular arrangement in crystals. For example, the velocity of light passing through a crystal is different in different directions. A ray of light entering in a crystal may split up into two components each following a different path and travelling with a different velocity.This phenomenon is called double refraction. In the figure two different kinds of atoms are shown in two dimensional arrangement. If the properties are measured along the direction CD, they will be different from those measured along the direction AB. This is due to the fact that in the direction AB each row is made up of one type of atoms while in the direction CD each row is made up of two types of atoms. It is important of note that in the case of
amorphous solids, liquids and gases atoms or molecules are indentical and all properties are same in all directions.
( v ) Cutting : Crystalline solids give clean cleavage while amorphous solids give irregular cut, due to conchoidal fracture on cutting with a sharp edged tool.
4 . CRYSTALLINE STATE :
"A crystal is a solid composed of atoms (ions or molecules) arranged in an orderly repetitive array".Most of the naturally occuring solids are found to have definite crystalline shapes which can be recognised easily. These are in large size because these are formed very slowly thus particles get sufficient time to get proper position in the crystal structure. Some crystalline solids are so small that appear to be amorphous.But on examination under a powerful microscope it is also seen to have a definite crystalline shape. Such solids are known as micro crystalline solide. Thus the crystallinity of a crystal may be defined as "a condition of matter resulting from an or orderly, cohesive, three dimensional arrangement of its component particles (atoms, ions or molecules) in space". This three dimensional arrangement is called crystal lattice or space lattice. The position occupied by the particles in the crystal lattice are called lattice sites or lattice points. The lattices are bound by surface that usually planar and known as faces of the crystal.
"T he smal le st geometrical posit ion of t he cr ystal wh ich can be used as repet it ive unit to bui ld up the whole crystal is called a unit cell."The angle between the two perpendiculars to the two intersecting faces is termed as the interfacial angle which may be same as the angle between the unit cell edges. Goniometer is used to measure the interfacial angle. It is important to note that interfacial angle of a substance remains the same although its shape may be different due to conditions of formation.
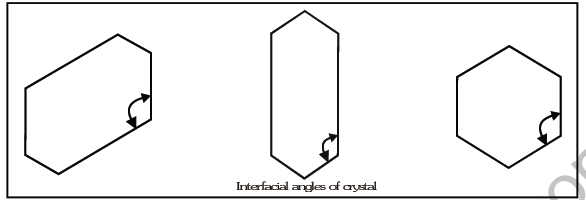
This is known as law of constancy of interfacial angle or law of crystallography.
5 . TYPES OF THE CRYSTALS :
Crystal are divided into four important types on the basis of chemical bonding of the constituent atoms.
( i ) Ionic Crystals :
These are formed by a combination of highely electro-positive ions (cations) and highly electronegative ions (anions). Thus strong electrostatic force of attraction acts with in the ionic crystals. Therefore, a large amount of energy is required to separate ions from one another. The type of the crystal lattice depends upon (i) The size of the ion (ii) The necessity for the preservation of electrical neutrality. Therefore alternate cations and anions in equivalent amounts are arranged in the ionic crystal e.g. NaCl, KF, CsCl etc.
( i i ) Covalent Crystals :
These are formed by sharing of valence electrons between two atoms resulting in the formation of a covalent bond. The covalent bonds extend in two or three dimensions forming a giant interlocking structure called network. Diamond and graphite are the good examples of this type.
( i i i ) Molecular Crystals :
In these crystals, molecules occupy the lattice points of the unit cells, except in solidified noble gases in which the units are atoms, where the binding is due to vander Waal's' forces and dipole-dipole forces. Since vander Waal's forces are non-directional hence structure of the crystal is determined by geometric consideration only. Solid H2, N2, O2, CO2, I2, sugar etc. are well known examples of such crystal in which vander Waal's forces are acting . Ice is the common example in which dipole-dipole forces of attraction (hydrogen bonding) are active. Many organic and inorganic crystals involve hydrogen bonds. Although these are comparatively weaker but play a very important role in determining the structures of substances e.g. polynucleoides, proteins etc.
( i v ) Metallic Crystals :
These are formed by a combination of atoms of electropositive elements. These atoms are binded by metallic bonds. It may be defined as :
The force that binds a metal ion to a number of electrons within its sphere of influences is known as metallic bond OR
A bond which is formed between electropositive elements OR
The attractive force which holds the atoms of two or more metals together in a metal crystal or in an alloy.

