♦ Introduction :
Complex organic compound which governs the common activities of the living organism are called biomolecules. Living systems are made up of various complex biomolecules like carbohydrates, proteins, nucleic acids, lipids, etc.
In addition, some simple molecules like vitamins and mineral salts also play an important role in the functions of organisms.
CARBOHYDRATES
Carbohydrates are primarily produced by plants and form a very large group of naturally occurring organic compounds. Some common examples are cane sugar, glucose, starch, etc. Most of them have a general formula, CX(H2O)y, and were considered as hydrates of carbon from where the name carbohydrate was derived. For example, the molecular formula of glucose (C6H12O6) fits into this general formula, C6(H2O)6. But all the compounds which fit into this formula may not be classified as carbohydrates. Rhamnose, C6H12O5 is a carbohydrate but does not fit in this definition. Chemically, the carbohydrates may be defined as optically act ive polyhy droxy aldehy de s or ketone s or t he compounds wh ich pro duce such unit s on hydrolysis. Some of the carbohydrates, which are sweet in taste, are also called sugars. The most common sugar, used in our homes is named as sucrose whereas the sugar present in milk is known as lactose.
♦ Classification of Carbohydrates :
Carbohydrates are classified on the basis of their behaviour on hydrolysis. They have been broadly divided into following three groups.
1 . Monosaccharides :
A carbohydrate that cannot be hydrolysed further to give simpler unit of polyhydroxy aldehyde or ketone is called a monosaccharide. Some common examples are glucose, fructose, ribose, etc.
Monosaccharides are further classified on the basis of number of carbon atoms and the functional group present in them. If a monosaccharide contains an aldehyde group, it is known as an aldose and if it contains a keto group, it is known as a ketose. Number of carbon atoms constituting the monosaccharide is also introduced in the name as is evident from the examples given in Table
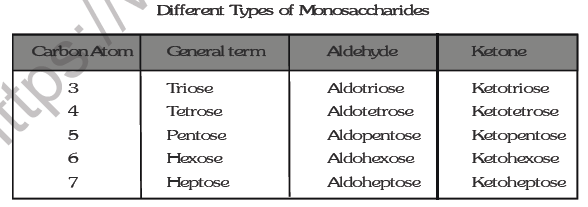
2 . Oligosaccharides :
Carbohydrates that yield two to ten monosaccharide units, on hydrolysis, are called oligosaccharides. They are further classified as disaccharides, trisaccharides, tetrasaccharides, etc., depending upon the number of monosaccharides, they provide on hydrolysis. Amongst these the most common are disaccharides. The two monosaccharide units obtained on hydrolysis of a disaccharide may be same or different.
For example, sucrose on hydrolysis gives one molecule each of glucose and fructose whereas maltose gives two molecules of glucose only.
3 . Polysaccharides :
Carbohydrates which yield a large number of monosaccharide units on hydrolysis are called polysaccharides. Some common examples are starch, cellulose, glycogen, gums, etc. Polysaccharides are not sweet in taste, hence they are also called non-sugars.
The carbohydrates may also be classified as either reducing or nonreducing sugars. All those carbohydrates which reduce Fehling’s solution and Tollens’ reagent are referred to as reducing sugars. All monosaccharides whether aldose or ketose are reduci ng sugar s.
In disaccharides, if the reducing groups of monosaccharides i.e., aldehydic or ketonic groups are bonded, these are non-reducing sugars e.g. sucrose. On the other hand, sugars in which these functional groups are free,are called reducing sugars, for example, maltose and lactose.
• Configuration in monosaccharides :
Glucose is correctly named as D(+)-glucose. ‘D’ before the name of glucose represents the configuration whereas ‘(+)’ represents dextrorotatory nature of the molecule. It may be remembered that ‘D’ and ‘L’ have no relation with the optical activity of the compound. The meaning of D– and L– notations is given as follows. The letters ‘D’ or ‘L’ before the name of any compound indicate the relative configuration of a particular stereoisomer. This refers to their relation with a particular isomer of glyceraldehyde. Glyceraldehyde contains one asymmetric carbon atom and exists in two enantiomeric forms as shown below.

All those compounds which can be chemically correlated to (+) isomer of glyceraldehyde are said to have D-configuration whereas those which can be correlated to (–) isomer of glyceraldehyde are said to have L- configuration. For assigning the configuration of monosaccharides, it is the lowest asymmetric carbon atom (as shown below) which is compared. As in (+) glucose, —OH on the lowest asymmetric carbon is on the right side which is comparable to (+) glyceraldehyde, so it is assigned D-configuration. For this comparison, the structure is written in a way that most oxidised carbon is at the top.
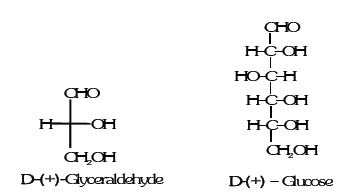
GLUCOSE (ALDOHEXOSE)
• Str ucture of Glucose :
Its molecular formula was found to be C6H12O6.
• Cyclic str ucture of Glucose :-
Glucose is found to exist in two different crystalline forms which are named as α and β. The α-form of glucose (m.p. 419 K) is obtained by crystallisation from concentrated solution of glucose at 303 K while the β-form (m.p. 423 K) is obtained by crystallisation from hot and saturated aqueous solution at 371 K.It was found that glucose forms a six-membered ring in which —OH at C-5 is involved in ring formation. This explains the absence of —CHO group and also existence of glucose in two forms as shown below. These two cyclic forms exist in equilibrium with open chain structure.

The two cyclic hemiacetal forms of glucose differ only in the configuration of the hydroxyl group at C1, called anomeric carbon (the aldehyde carbon before cyclisation). Such isomers, i.e., α-form and β-form, are called anomers. The six membered cyclic structure of glucose is called pyranose structure (α– or β–), in analogy with pyran. Pyran is a cyclic organic compound with one oxygen atom and five carbon atoms in the ring. The cyclic structure of glucose is more correctly represented by Haworth structure as given below.

