NCERT Solutions Class 9 Science Chapter 3 Atoms and Molecules have been provided below and is also available in Pdf for free download. The NCERT solutions for Class 9 Science have been prepared as per the latest syllabus, NCERT books and examination pattern suggested in Class 9 by CBSE, NCERT and KVS. Questions given in NCERT book for Class 9 Science are an important part of exams for Class 9 Science and if answered properly can help you to get higher marks. Refer to more Chapter-wise answers for NCERT Class 9 Science and also download more latest study material for all subjects. Chapter 3 Atoms and Molecules is an important topic in Class 9, please refer to answers provided below to help you score better in exams
Chapter 3 Atoms and Molecules Class 9 Science NCERT Solutions
Class 9 Science students should refer to the following NCERT questions with answers for Chapter 3 Atoms and Molecules in Class 9. These NCERT Solutions with answers for Class 9 Science will come in exams and help you to score good marks
Chapter 3 Atoms and Molecules NCERT Solutions Class 9 Science
Class IX
Chapter 3 – Atoms and Molecules Science
Question 1: In a reaction, 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass.
Sodium carbonate + ethanoic acid → sodium ethanoate + carbon dioxide + water
Answer: In the given reaction, sodium carbonate reacts with ethanoic acid to produce sodium ethanoate, carbon dioxide, and water.
Mass of sodium carbonate = 5.3 g (Given)
Mass of ethanoic acid = 6 g (Given)
Mass of sodium ethanoate = 8.2 g (Given)
Mass of carbon dioxide = 2.2 g (Given)
Mass of water = 0.9 g (Given)
Now, total mass before the reaction = (5.3 + 6) g
= 11.3 g
And, total mass after the reaction = (8.2 + 2.2 + 0.9) g
= 11.3 g
∴Total mass before the reaction = Total mass after the reaction
Hence, the given observations are in agreement with the law of conservation of mass.
Question 2: Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?Answer:
It is given that the ratio of hydrogen and oxygen by mass to form water is 1:8. Then, the mass of oxygen gas required to react completely with 1 g of hydrogen gas is 8 g. Therefore, the mass of oxygen gas required to react completely with 3 g of hydrogen gas is 8 × 3 g = 24 g.
Question 3: Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Answer: The postulate of Dalton’s atomic theory which is a result of the law of conservation of mass is:
Atoms are indivisible particles, which can neither be created nor destroyed in a chemical reaction.
Question 4: Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Answer: The postulate of Dalton’s atomic theory which can explain the law of definitevproportion is: The relative number and kind of atoms in a given compound remains constant.
Chapter 3 – Atoms and Molecules
Question 1: Define atomic mass unit.
Answer: Mass unit equal to exactly one-twelfth (1/12th) the mass of one atom of carbon-12 is called one atomic mass unit. It is written as ‘u’.
Question 2: Why is it not possible to see an atom with naked eyes?
Answer: Thesize of an atom is so small that it is not possible to see it with naked eyes. Also, the atom of an element does not exist independently.
Chapter 3 – Atoms and Molecules
Question 1: Write down the formulae of
(i) sodium oxide
(ii) aluminium chloride
(iii) sodium suphide
(iv) magnesium hydroxide
Answer:
(i) Sodium oxide →Na2O
(ii) Aluminium chloride → AlCl3
(iii) Sodium suphide → Na2S
(iv) Magnesium hydroxide → Mg(OH)2
Question 2: Write down the names of compounds represented by the following formulae:
(i) Al2(SO4)3
(ii) CaCl2
(iii) K2SO4
(iv) KNO3
(v) CaCO3
Answer:
(i) Al2(SO4)3 → Aluminium sulphate
(ii) CaCl2 → Calcium chloride
(iii) K2SO4 → Potassium sulphate
(iv) KNO3 → Potassium nitrate
(v) CaCO3 → Calcium carbonate
Question 3: What is meant by the term chemical formula?
Answer: The chemical formula of a compound means the symbolic representation of the composition of a compound. From the chemical formula of a compound, we can know the number and kinds of atoms of different elements that constitute the compound. For example, from the chemical formula CO2 of carbon dioxide, we come to know that one carbon atom and two oxygen atoms are chemically bonded together to form one molecule of the compound, carbon dioxide.
Question 4: How many atoms are present in a
(i) H2S molecule and
(ii) PO43− ion?
Answer: (i) In an H2S molecule, three atoms are present; two of hydrogen and one of sulphur.
(ii) In a PO43− ion, five atoms are present; one of phosphorus and four of oxygen.
Chapter 3 – Atoms and Molecules
Question 1: Calculate the molecular masses of H2, O2, Cl2, CO2, CH4, C2H6, C2H4, NH3, CH3OH.
Answer:
Molecular mass of H2 = 2 × Atomic mass of H
= 2 × 1
= 2 u
Molecular mass of O2 = 2 × Atomic mass of O
= 2 × 16
= 32 u
Molecular mass of Cl2 = 2 × Atomic mass of Cl
= 2 × 35.5
= 71 u
Molecular mass of CO2 = Atomic mass of C + 2 × Atomic mass of O
= 12 + 2 × 16
= 44 u
Molecular mass of CH4 = Atomic mass of C + 4 × Atomic mass of H
= 12 + 4 × 1
= 16 u
Molecular mass of C2H6 = 2 × Atomic mass of C + 6 × Atomic mass of H
= 2 × 12 + 6 × 1
= 30 u
Molecular mass of C2H4 = 2 × Atomic mass of C + 4 × Atomic mass of H
= 2 × 12 + 4 × 1
= 28 u
Molecular mass of NH3 = Atomic mass of N + 3 × Atomic mass of H
= 14 + 3 × 1
= 17 u
Molecular mass of CH3OH = Atomic mass of C + 4 × Atomic mass of H + Atomic mass of O
= 12 + 4 × 1 + 16
= 32 u
Question 2:
Calculate the formula unit masses of ZnO, Na2O, K2CO3, given atomic masses of Zn
= 65 u, Na = 23 u, K = 39 u, C = 12 u, and O = 16 u.
Answer:
Formula unit mass of ZnO = Atomic mass of Zn + Atomic mass of O
= 65 + 16
= 81 u
Formula unit mass of Na2O = 2 × Atomic mass of Na + Atomic mass of O
= 2 × 23 + 16
= 62 u
Formula unit mass of K2CO3 = 2 × Atomic mass of K + Atomic mass of C + 3 × Atomic mass of O
= 2 × 39 + 12 + 3 × 16
= 138 u
Chapter 3 – Atoms and Molecules
Question 1: If one mole of carbon atoms weighs 12 gram, what is the mass (in gram) of 1 atom of carbon?
Answer:
One mole of carbon atoms weighs 12 g (Given)
i.e., mass of 1 mole of carbon atoms = 12 g
Then, mass of 6.022x1023 number of carbon atoms = 12 g
Therefore, mass of 1 atom of carbon = 12/6.022x1023g = 1.9926 x 10-23g
Question 2: Which has more number of atoms, 100 grams of sodium or 100 grams of iron (given, atomic mass of Na = 23 u, Fe = 56 u)?
Answer:
Atomic mass of Na = 23 u (Given)
Then, gram atomic mass of Na = 23 g
Now, 23 g of Na contains 6.022x1023 = number of atoms
Thus, 100 g of Na contains 6.022x1023/23 x 100 number of atoms
= 2.61826 x 1024number of atoms
Again, atomic mass of Fe = 56 u(Given)
Then, gram atomic mass of Fe = 56 g
Now, 56 g of Fe contains 6.022x1023= number of atoms
Thus, 100 g of Fe contains 6.022x1023/56 x 100 number of atoms
= 1.0753 x 1024number of atoms
Therefore, 100 grams of sodium contain more number of atoms than 100 grams of iron.
Chapter 3 – Atoms and Molecules
Question 1: A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Answer:
Mass of boron = 0.096 g (Given)
Mass of oxygen = 0.144 g (Given)
Mass of sample = 0.24 g (Given)
Thus, percentage of boron by weight in the compound = 0.096 /0.24 x 100%
= 40%
And, percentage of oxygen by weight in the compound = 0.144/0.24 x 100%
= 60%
Question 2: When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combinations will govern your answer?
Answer: Carbon + Oxygen → Carbon dioxide
3 g of carbon reacts with 8 g of oxygen to produce 11 g of carbon dioxide.
If 3 g of carbon is burnt in 50 g of oxygen, then 3 g of carbon will react with 8 g of oxygen. The remaining 42 g of oxygen will be left un-reactive.
In this case also, only 11 g of carbon dioxide will be formed.
The above answer is governed by the law of constant proportions.
Question 3: What are polyatomic ions? Give examples?
Answer: A polyatomic ion is a group of atoms carrying a charge (positive or negative). For example, ammonium ion (NH+4) , hydroxide ion (OH−), carbonate ion (CO2-3) sulphate ion (SO2-4)
Question 4: Write the chemical formulae of the following:
(a) Magnesium chloride
(b) Calcium oxide
(c) Copper nitrate
(d) Aluminium chloride
(e) Calcium carbonate
Answer:
(a) Magnesium chloride → MgCl2
(b) Calcium oxide → CaO
(c) Copper nitrate → Cu (NO3)2
(d) Aluminium chloride → AlCl3
(e) Calcium carbonate → CaCO3
Question 5: Give the names of the elements present in the following compounds:
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium sulphate
Answer:
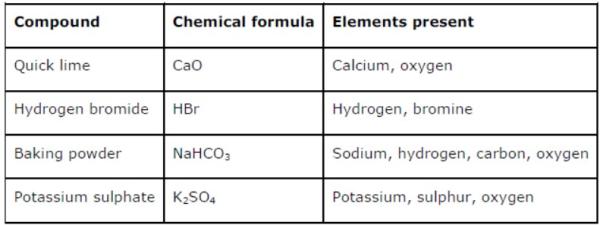
Question 6: Calculate the molar mass of the following substances:
(a) Ethyne, C2H2
(b) Sulphur molecule, S8
(c) Phosphorus molecule, P4 (atomic mass of phosphorus = 31)
(d) Hydrochloric acid, HCl
(e) Nitric acid, HNO3
Answer:
(a) Molar mass of ethyne, C2H2 = 2 × 12 + 2 × 1 = 28 g
(b) Molar mass of sulphur molecule, S8 = 8 × 32 = 256 g
(c) Molar mass of phosphorus molecule, P4 = 4 × 31 = 124 g
(d) Molar mass of hydrochloric acid, HCl = 1 + 35.5 = 36.5 g
(e) Molar mass of nitric acid, HNO3 = 1 + 14 + 3 × 16 = 63 g
Question 7: What is the mass of−−
(a) 1 mole of nitrogen atoms?
(b) 4 moles of aluminium atoms (Atomic mass of aluminium = 27)?
(c) 10 moles of sodium sulphite (Na2SO3)?
Answer:
(a) The mass of 1 mole of nitrogen atoms is 14 g.
(b) The mass of 4 moles of aluminium atoms is (4 × 27) g = 108 g
(c) The mass of 10 moles of sodium sulphite (Na2SO3) is
10 × [2 × 23 + 32 + 3 × 16] g = 10 × 126 g = 1260 g
Question 8: Convert into mole.
(a) 12 g of oxygen gas
(b) 20 g of water
(c) 22 g of carbon dioxide
Answer:
(a) 32 g of oxygen gas = 1 mole
Then, 12 g of oxygen gas = 12/32 mole = 0.375 mole
(b) 18 g of water = 1 mole
Then, 20 g of water = 20/18 mole = 1.11 moles (approx)
(c) 44 g of carbon dioxide = 1 mole
Then, 22 g of carbon dioxide = 22/44 mole = 0.5 mole
Question 9: What is the mass of:
(a) 0.2 mole of oxygen atoms?
(b) 0.5 mole of water molecules?
Answer:
(a) Mass of one mole of oxygen atoms = 16 g
Then, mass of 0.2 mole of oxygen atoms = 0.2 × 16g = 3.2 g
(b) Mass of one mole of water molecule = 18 g
Then, mass of 0.5 mole of water molecules = 0.5 × 18 g = 9 g
Question 10: Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur.
Answer:
1 mole of solid sulphur (S8) = 8 × 32 g = 256 g
i.e., 256 g of solid sulphur contains = 6.022 × 1023 molecules
Then, 16 g of solid sulphur contains = 6.022 x 1023 /256 x 16 molecules
= 3.76 × 1022 molecules (approx)
Question 11: Calculate the number of aluminium ions present in 0.051 g of aluminium oxide.
(Hint: The mass of an ion is the same as that of an atom of the same element.
Atomic mass of Al = 27 u)
Answer:
1 mole of aluminium oxide (Al2O3) = 2 × 27 + 3 × 16 = 102 g
i.e., 102 g of Al2O3 = 6.022 × 1023 molecules of Al2O3
Then, 0.051 g of Al2O3 contains = 6.022 x 1023 /102 x 0.051 molecules
= 3.011 × 1020 molecules of Al2O3
The number of aluminium ions (Al3+) present in one molecule of aluminium oxide is 2.
Therefore, the number of aluminium ions (Al3+) present in 3.011 × 1020 molecules
(0.051 g ) of aluminium oxide (Al2O3) = 2 × 3.011 × 1020
= 6.022 × 1020
| NCERT Solutions Class 9 Science Chapter 1 Matter in Our Surroundings |
| NCERT Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure |
| NCERT Solutions Class 9 Science Chapter 3 Atoms and Molecules |
| NCERT Solutions Class 9 Science Chapter 4 Structure of the Atom |
| NCERT Solutions Class 9 Science Chapter 5 The Fundamental Unit of Life |
| NCERT Solutions Class 9 Science Chapter 6 Tissues |
| NCERT Solutions Class 9 Science Chapter 7 Diversity in Living Organisms |
| NCERT Solutions Class 9 Science Chapter 8 Motion |
| NCERT Solutions Class 9 Science Chapter 9 Force and Laws of Motion |
| NCERT Solutions Class 9 Science Chapter 10 Gravitation |
| NCERT Solutions Class 9 Science Chapter 11 Work and Energy |
| NCERT Solutions Class 9 Science Chapter 12 Sound |
| NCERT Solutions Class 9 Science Chapter 13 Why Do We Fall Ill |
| NCERT Solutions Class 9 Science Chapter 14 Natural Resources |
| NCERT Solutions Class 9 Science Chapter 15 Improvement in Food Resources |
More Study Material
NCERT Solutions Class 9 Science Chapter 3 Atoms and Molecules
NCERT Solutions Class 9 Science Chapter 3 Atoms and Molecules is available on our website www.studiestoday.com for free download in Pdf. You can read the solutions to all questions given in your Class 9 Science textbook online or you can easily download them in pdf.
Chapter 3 Atoms and Molecules Class 9 Science NCERT Solutions
The Class 9 Science NCERT Solutions Chapter 3 Atoms and Molecules are designed in a way that will help to improve the overall understanding of students. The answers to each question in Chapter 3 Atoms and Molecules of Science Class 9 has been designed based on the latest syllabus released for the current year. We have also provided detailed explanations for all difficult topics in Chapter 3 Atoms and Molecules Class 9 chapter of Science so that it can be easier for students to understand all answers.
NCERT Solutions Chapter 3 Atoms and Molecules Class 9 Science
Class 9 Science NCERT Solutions Chapter 3 Atoms and Molecules is a really good source using which the students can get more marks in exams. The same questions will be coming in your Class 9 Science exam. Learn the Chapter 3 Atoms and Molecules questions and answers daily to get a higher score. Chapter 3 Atoms and Molecules of your Science textbook has a lot of questions at the end of chapter to test the students understanding of the concepts taught in the chapter. Students have to solve the questions and refer to the step-by-step solutions provided by Science teachers on studiestoday to get better problem-solving skills.
Chapter 3 Atoms and Molecules Class 9 NCERT Solution Science
These solutions of Chapter 3 Atoms and Molecules NCERT Questions given in your textbook for Class 9 Science have been designed to help students understand the difficult topics of Science in an easy manner. These will also help to build a strong foundation in the Science. There is a combination of theoretical and practical questions relating to all chapters in Science to check the overall learning of the students of Class 9.
Class 9 NCERT Solution Science Chapter 3 Atoms and Molecules
NCERT Solutions Class 9 Science Chapter 3 Atoms and Molecules detailed answers are given with the objective of helping students compare their answers with the example. NCERT solutions for Class 9 Science provide a strong foundation for every chapter. They ensure a smooth and easy knowledge of Revision notes for Class 9 Science. As suggested by the HRD ministry, they will perform a major role in JEE. Students can easily download these solutions and use them to prepare for upcoming exams and also go through the Question Papers for Class 9 Science to clarify all doubts
You can download the NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules for latest session from StudiesToday.com
Yes, you can click on the link above and download NCERT Solutions in PDFs for Class 9 for Science Chapter 3 Atoms and Molecules
Yes, the NCERT Solutions issued for Class 9 Science Chapter 3 Atoms and Molecules have been made available here for latest academic session
You can easily access the links above and download the Chapter 3 Atoms and Molecules Class 9 NCERT Solutions Science for each chapter
There is no charge for the NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules you can download everything free
Regular revision of NCERT Solutions given on studiestoday for Class 9 subject Science Chapter 3 Atoms and Molecules can help you to score better marks in exams
Yes, studiestoday.com provides all latest NCERT Chapter 3 Atoms and Molecules Class 9 Science solutions based on the latest books for the current academic session
Yes, studiestoday provides NCERT solutions for Chapter 3 Atoms and Molecules Class 9 Science in mobile-friendly format and can be accessed on smartphones and tablets.
Yes, NCERT solutions for Class 9 Chapter 3 Atoms and Molecules Science are available in multiple languages, including English, Hindi

